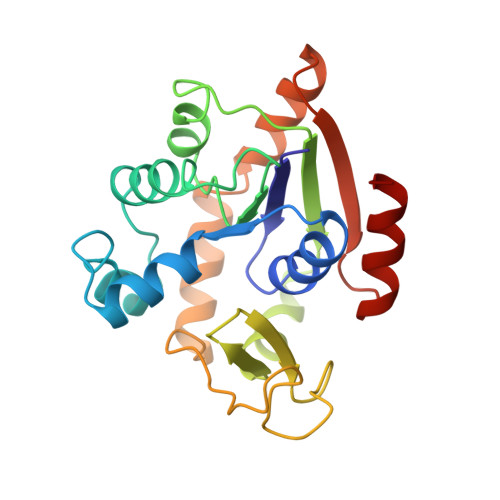
The closed conformation of a highly flexible protein: the structure of E. coli adenylate kinase with bound AMP and AMPPNP.
Berry, M.B., Meador, B., Bilderback, T., Liang, P., Glaser, M., Phillips Jr., G.N.(1994) Proteins 19: 183-198
- PubMed: 7937733
- DOI: https://doi.org/10.1002/prot.340190304
- Primary Citation of Related Structures:
1ANK - PubMed Abstract:
The structure of E. coli adenylate kinase with bound AMP and AMPPNP at 2.0 A resolution is presented. The protein crystallizes in space group C2 with two molecules in the asymmetric unit, and has been refined to an R factor of 20.1% and an Rfree of 31.6%. In the present structure, the protein is in the closed (globular) form with the large flexible lid domain covering the AMPPNP molecule. Within the protein, AMP and AMPPNP, and ATP analog, occupy the AMP and ATP sites respectively, which had been suggested by the most recent crystal structure of E. coli adenylate kinase with Ap5A bound (Müller and Schulz, 1992, ref. 1) and prior fluorescence studies (Liang et al., 1991, ref. 2). The binding of substrates and the positions of the active site residues are compared between the present structure and the E. coli adenylate kinase/Ap5A structure. We failed to detect a peak in the density map corresponding to the Mg2+ ion which is required for catalysis, and its absence has been attributed to the use of ammonium sulfate in the crystallization solution. Finally, a comparison is made between the present structure and the structure of the heavy chain of muscle myosin.
- W.M. Keck Center for Computational Biology, Rice University, Houston, Texas 77251-1892.
Organizational Affiliation: