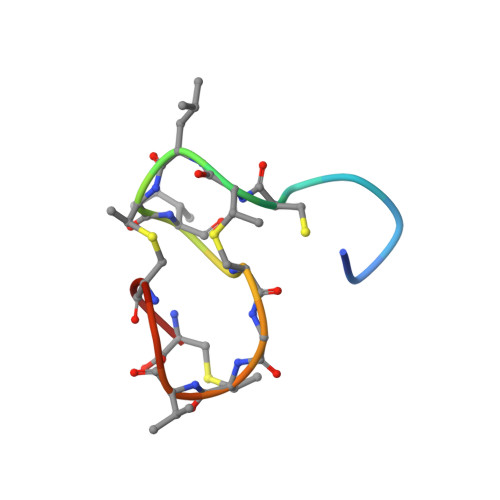
The three-dimensional solution structure of the lantibiotic murein-biosynthesis-inhibitor actagardine determined by NMR.
Zimmermann, N., Jung, G.(1997) Eur J Biochem 246: 809-819
- PubMed: 9219543
- DOI: https://doi.org/10.1111/j.1432-1033.1997.00809.x
- Primary Citation of Related Structures:
1AJ1 - PubMed Abstract:
The three-dimensional solution structure of the lantibiotic actagardine was determined at high resolution by homonuclear and heteronuclear two-dimensional and three-dimensional NMR spectroscopy in [2H3]acetonitrile/H2O (7:3). 133 non-trivial distance and 22 torsional-angle constraints were derived from the NMR data. An ensemble of 15 low-energy structures was calculated by distance geometry followed by an iterative relaxation-matrix-refinement procedure. The rmsd of the backbone coordinates with respect to the average structure was 17 pm. The two distinct thioether ring systems 1-6 and 7-19 were even better defined, with backbone rmsd of 10 pm and 14 pm, respectively. Actagardine shows a rigid compact globular shape based on the constraining bridging pattern, which is composed of an N-terminal lanthionine ring from residues 1-6 and three intertwined C-terminal methyllanthionine rings comprising residues 7-12, 9-17 and 14-19. In addition, this C-terminal ring system is stabilised by a short antiparallel beta sheet. A feature of the actagardine structure is the presence of two putative binding pockets. A pocket is generated by the covalent constraints of the C-terminal thioether ring system. The rim of this pocket is built up by a loop structure comprising residues 12-19, whose backbone amide protons are all directed to the centre of the pocket. The second pocket is formed by an L-shaped orientation of the N-terminal and C-terminal thioether ring systems. The only two hydrophilic amino acid residues of actagardine, Glu11 and Ser2, are directed to this pocket. A region of high sequence similarity with the related lantibiotic mersacidin is located exactly at the position of the second pocket (residues 3-12). This suggests that the second pocket is responsible for the antibiotic mode of action of actagardine and mersacidin as inhibitors of the murein biosynthesis of gram-positive bacteria.
- Institut für Organische Chemie, Universität Tübingen, Germany.
Organizational Affiliation: