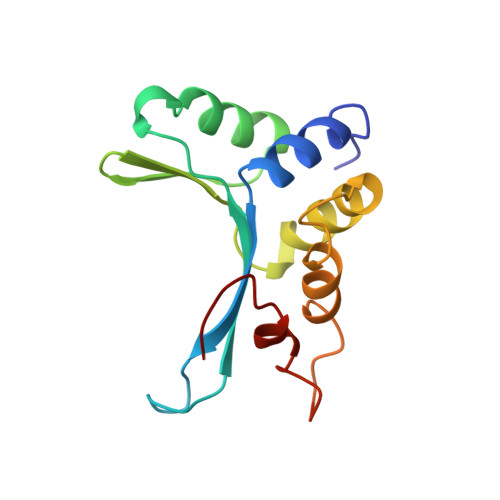
The structure of I-Crel, a group I intron-encoded homing endonuclease.
Heath, P.J., Stephens, K.M., Monnat Jr., R.J., Stoddard, B.L.(1997) Nat Struct Biol 4: 468-476
- PubMed: 9187655
- DOI: https://doi.org/10.1038/nsb0697-468
- Primary Citation of Related Structures:
1AF5 - PubMed Abstract:
The structure of I-Crel provides the first view of a protein encoded by a gene within an intron. This endonuclease recognizes a long DNA site approximately 20 base pairs in length and facilitates the lateral transfer of that intron. The protein exhibits a DNA-binding surface consisting of four antiparallel beta-strands that form a 20 A wide groove which is over 70 A long. The architecture of this fold is different from that of the TATA binding protein, TBP, which also contains an antiparallel beta-saddle. The conserved LAGLIDADG motif, which is found in many mobile intron endonucleases, maturases and inteins, forms a novel helical interface and contributes essential residues to the active site.
- Fred Hutchinson Cancer Research Center, Division of Basic Sciences, Seattle, Washington 98104, USA.
Organizational Affiliation: