ATP induced conformational changes facilitate E1-E2 disulfide bridging in the ubiquitin system.
Misra, M., Schaefer, A., Kuhn, M., Pluska, L., Tessmer, I., Sommer, T., Schindelin, H.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Ubiquitin-activating enzyme E1 1 | A [auth C] | 1,001 | Saccharomyces cerevisiae S288C | Mutation(s): 0 Gene Names: UBA1, YKL210W EC: 6.2.1.45 | 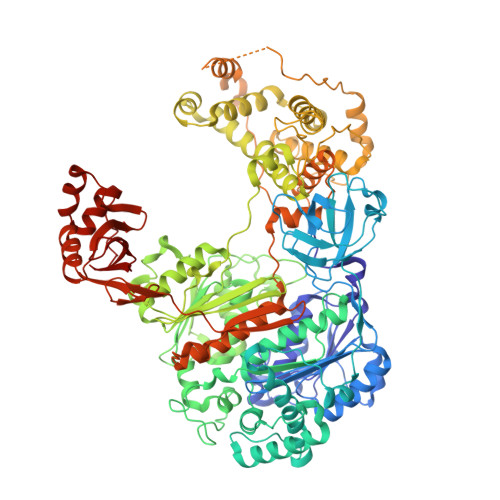 |
UniProt | |||||
Find proteins for P22515 (Saccharomyces cerevisiae (strain ATCC 204508 / S288c)) Explore P22515 Go to UniProtKB: P22515 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P22515 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Ubiquitin-conjugating enzyme E2 13 | 153 | Saccharomyces cerevisiae S288C | Mutation(s): 0 Gene Names: UBC13, YDR092W, YD6652.04 EC: 2.3.2.23 | 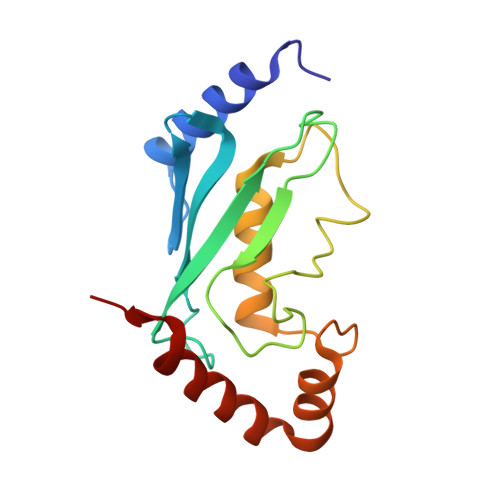 | |
UniProt | |||||
Find proteins for P52490 (Saccharomyces cerevisiae (strain ATCC 204508 / S288c)) Explore P52490 Go to UniProtKB: P52490 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P52490 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| GOL Query on GOL | C D [auth C] E [auth C] F [auth C] G [auth C] | GLYCEROL C3 H8 O3 PEDCQBHIVMGVHV-UHFFFAOYSA-N |  | ||
| CL Query on CL | N [auth C], O [auth C], P [auth C], Q [auth C] | CHLORIDE ION Cl VEXZGXHMUGYJMC-UHFFFAOYSA-M |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 181.39 | α = 90 |
| b = 58.27 | β = 109.72 |
| c = 137.46 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| XDS | data reduction |
| Aimless | data scaling |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| German Research Foundation (DFG) | Germany | SCHI 425-6/1 |