Engineered C-N Lyase: Enantioselective Synthesis of Chiral Synthons for Artificial Dipeptide Sweeteners.
Zhang, J., Grandi, E., Fu, H., Saravanan, T., Bothof, L., Tepper, P.G., Thunnissen, A.W.H., Poelarends, G.J.(2020) Angew Chem Int Ed Engl 59: 429-435
- PubMed: 31625664
- DOI: https://doi.org/10.1002/anie.201910704
- Primary Citation of Related Structures:
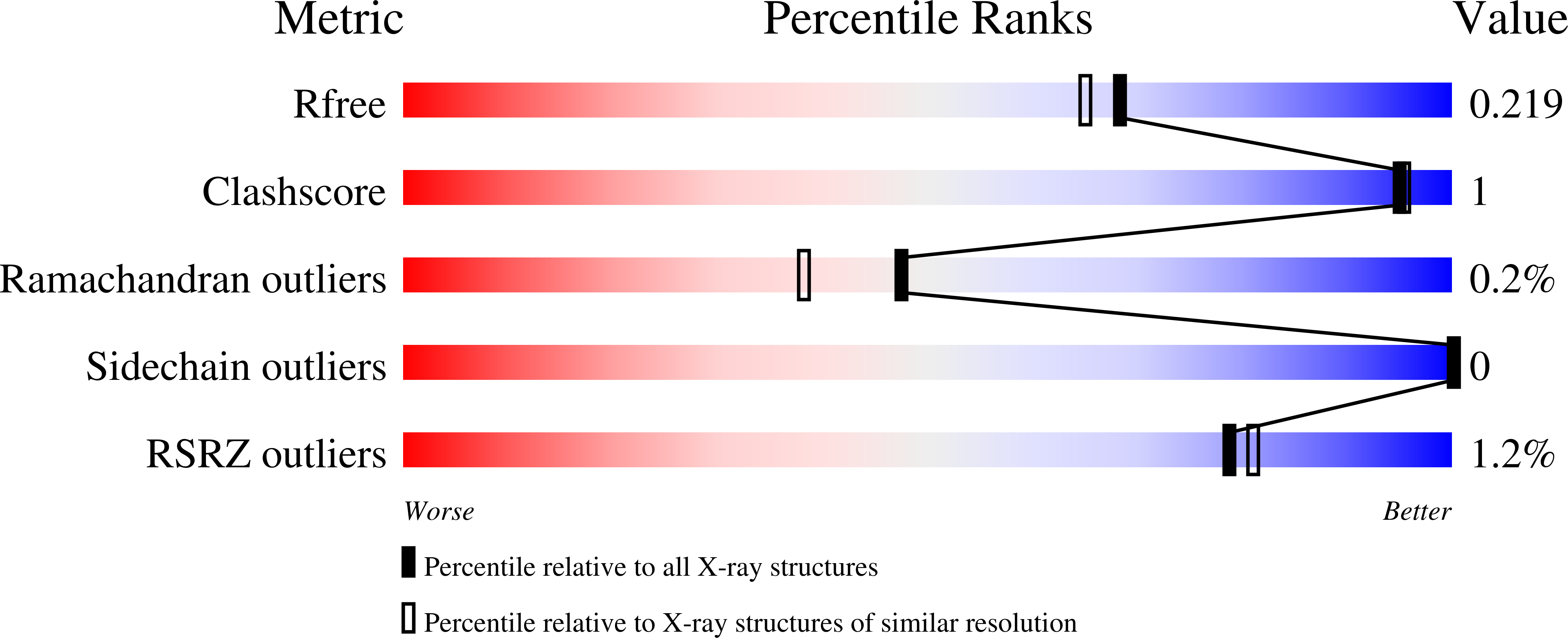
6RX8, 6RXA - PubMed Abstract:
Aspartic acid derivatives with branched N-alkyl or N-arylalkyl substituents are valuable precursors to artificial dipeptide sweeteners such as neotame and advantame. The development of a biocatalyst to synthesize these compounds in a single asymmetric step is an as yet unmet challenge. Reported here is an enantioselective biocatalytic synthesis of various difficult N-substituted aspartic acids, including N-(3,3-dimethylbutyl)-l-aspartic acid and N-[3-(3-hydroxy-4-methoxyphenyl)propyl]-l-aspartic acid, precursors to neotame and advantame, respectively, using an engineered variant of ethylenediamine-N,N'-disuccinic acid (EDDS) lyase from Chelativorans sp. BNC1. This engineered C-N lyase (mutant D290M/Y320M) displayed a remarkable 1140-fold increase in activity for the selective hydroamination of fumarate compared to that of the wild-type enzyme. These results present new opportunities to develop practical multienzymatic processes for the more sustainable and step-economic synthesis of an important class of food additives.
Organizational Affiliation:
Department of Chemical and Pharmaceutical Biology, Groningen Research Institute of Pharmacy, University of Groningen, Antonius Deusinglaan 1, 9713, AV, Groningen, The Netherlands.