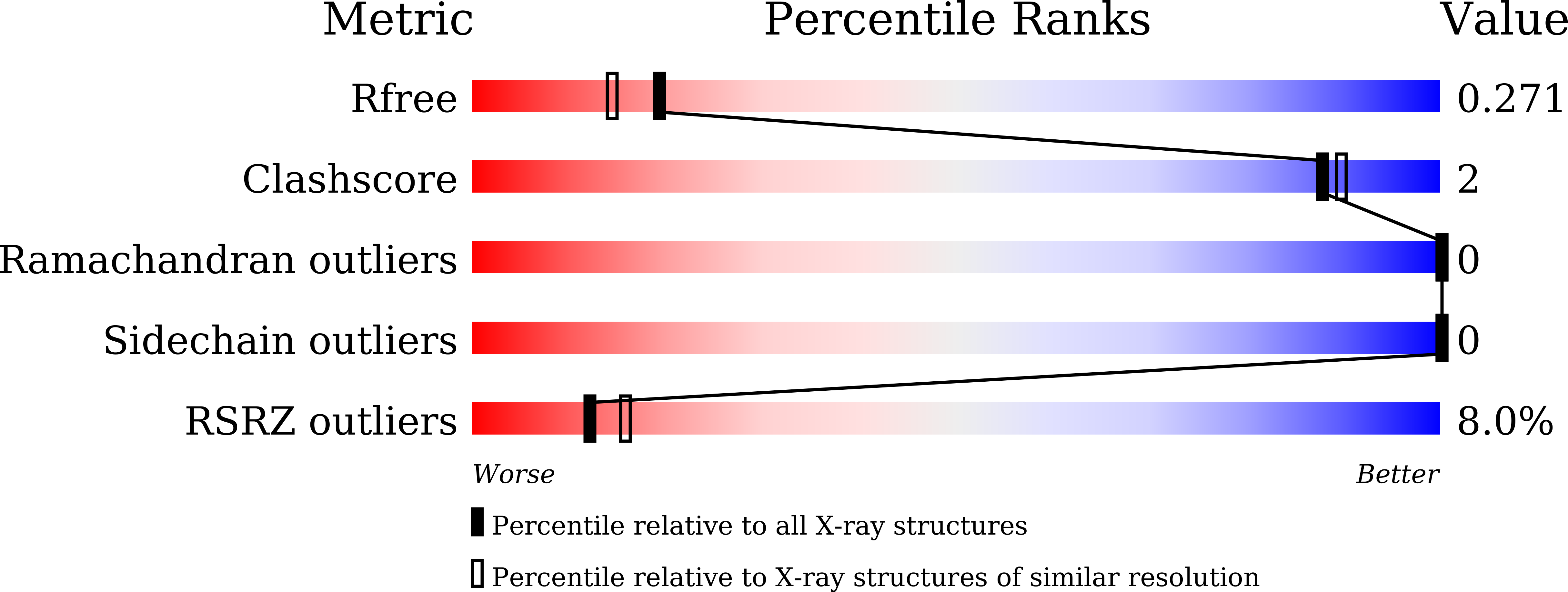
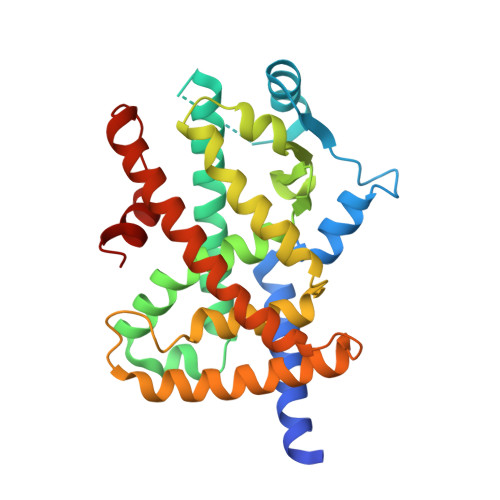
Cyclin-Dependent Kinase 5 Inhibitor Butyrolactone I Elicits a Partial Agonist Activity of Peroxisome Proliferator-Activated Receptor gamma.
Ahn, S., Jang, D.M., Park, S.C., An, S., Shin, J., Han, B.W., Noh, M.(2020) Biomolecules 10
- PubMed: 32054125
- DOI: https://doi.org/10.3390/biom10020275
- Primary Citation of Related Structures:
6L89, 6L8B - PubMed Abstract:
Adiponectin is an adipocyte-derived cytokine having an insulin-sensitizing activity. During the phenotypic screening of secondary metabolites derived from the marine fungus Aspergillus terreus , a poly cyclin-dependent kinase (CDK) inhibitor butyrolactone I affecting CDK1 and CDK5 was discovered as a potent adiponectin production-enhancing compound in the adipogenesis model of human bone marrow-derived mesenchymal stem cells (hBM-MSCs). CDK5 inhibitors exhibit insulin-sensitizing activities by suppressing the phosphorylation of peroxisome proliferator-activated receptor γ (PPARγ). However, the adiponectin production-enhancing activities of butyrolactone I have not been correlated with the potency of CDK5 inhibitor activities. In a target identification study, butyrolactone I was found to directly bind to PPARγ. In the crystal structure of the human PPARγ, the ligand-binding domain (LBD) in complex with butyrolactone I interacted with the amino acid residues located in the hydrophobic binding pockets of the PPARγ LBD, which is a typical binding mode of the PPARγ partial agonists. Therefore, the adiponectin production-enhancing effect of butyrolactone I was mediated by its polypharmacological dual modulator activities as both a CDK5 inhibitor and a PPARγ partial agonist.
Organizational Affiliation:
Natural Products Research Institute, College of Pharmacy, Seoul National University, 1 Gwanak-ro, Gwanak-gu, Seoul 08826, Korea.