Crystal structure of the complex of the interaction domains of Escherichia coli DnaB helicase and DnaC helicase loader: structural basis implying a distortion-accumulation mechanism for the DnaB ring opening caused by DnaC binding.
Nagata, K., Okada, A., Ohtsuka, J., Ohkuri, T., Akama, Y., Sakiyama, Y., Miyazaki, E., Horita, S., Katayama, T., Ueda, T., Tanokura, M.(2020) J Biochem 167: 1-14
- PubMed: 31665315
- DOI: https://doi.org/10.1093/jb/mvz087
- Primary Citation of Related Structures:
6KZA - PubMed Abstract:
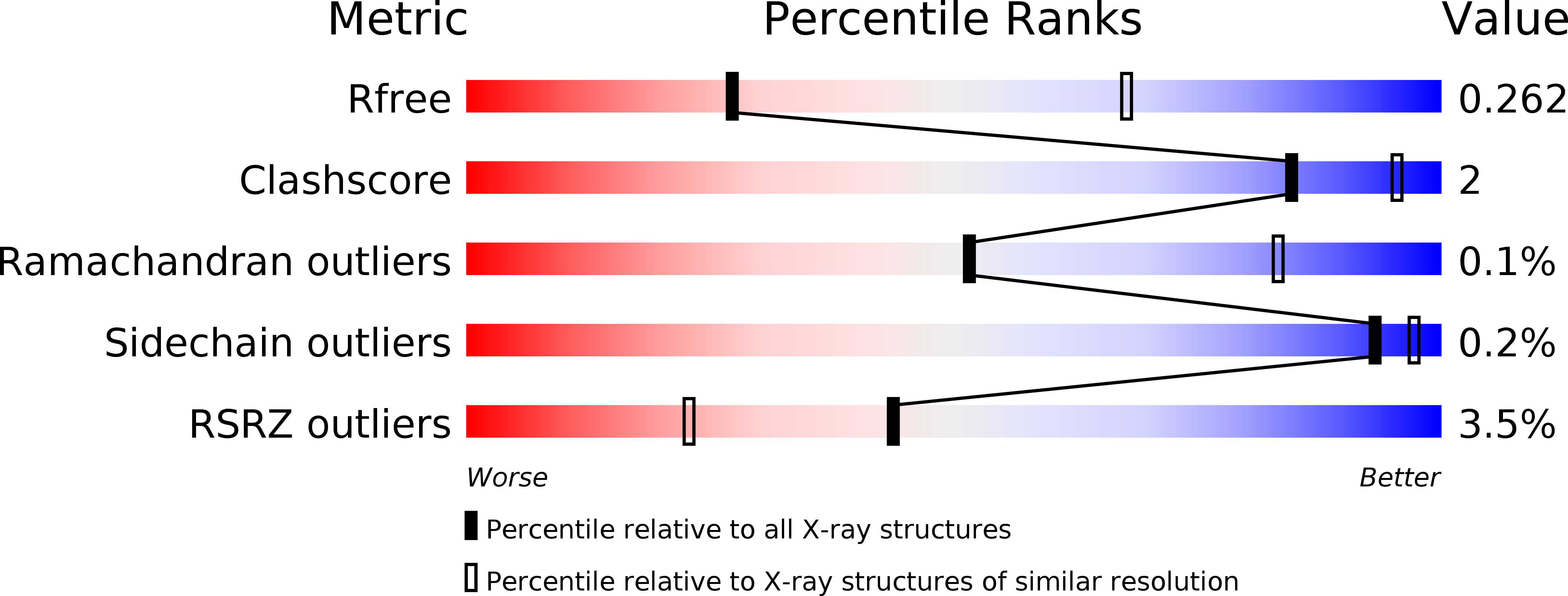
Loading the bacterial replicative helicase DnaB onto DNA requires a specific loader protein, DnaC/DnaI, which creates the loading-competent state by opening the DnaB hexameric ring. To understand the molecular mechanism by which DnaC/DnaI opens the DnaB ring, we solved 3.1-Å co-crystal structure of the interaction domains of Escherichia coli DnaB-DnaC. The structure reveals that one N-terminal domain (NTD) of DnaC interacts with both the linker helix of a DnaB molecule and the C-terminal domain (CTD) of the adjacent DnaB molecule by forming a three α-helix bundle, which fixes the relative orientation of the two adjacent DnaB CTDs. The importance of the intermolecular interface in the crystal structure was supported by the mutational data of DnaB and DnaC. Based on the crystal structure and other available information on DnaB-DnaC structures, we constructed a molecular model of the hexameric DnaB CTDs bound by six DnaC NTDs. This model suggested that the binding of a DnaC would cause a distortion in the hexameric ring of DnaB. This distortion of the DnaB ring might accumulate by the binding of up to six DnaC molecules, resulting in the DnaB ring to open.
Organizational Affiliation:
Department of Applied Biological Chemistry, Graduate School of Agricultural and Life Sciences, The University of Tokyo, Bunkyo-ku, Tokyo, Japan.