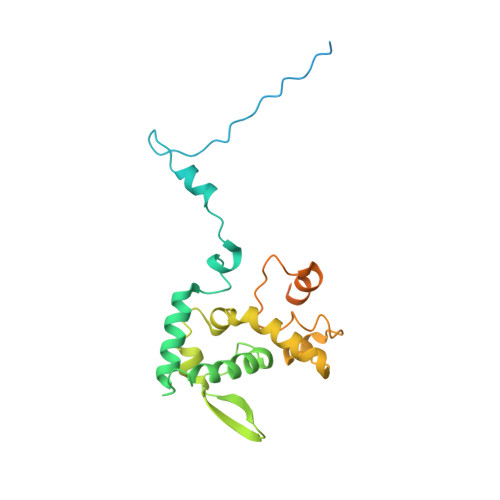
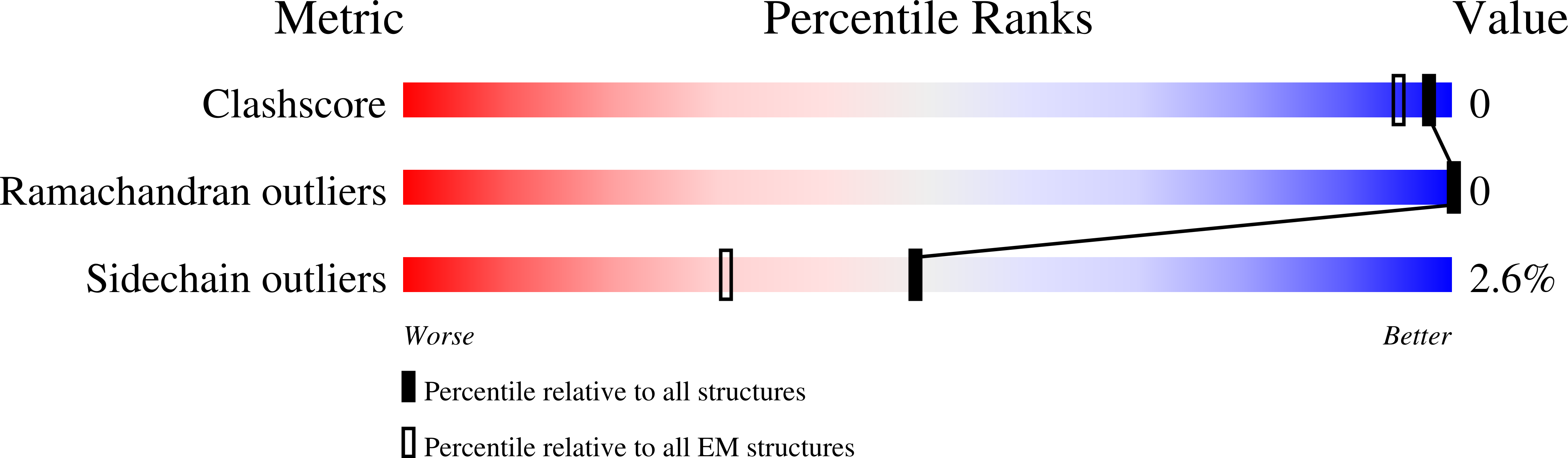Structural basis for the multitasking nature of the potato virus Y coat protein.
Kezar, A., Kavcic, L., Polak, M., Novacek, J., Gutierrez-Aguirre, I., Znidaric, M.T., Coll, A., Stare, K., Gruden, K., Ravnikar, M., Pahovnik, D., Zagar, E., Merzel, F., Anderluh, G., Podobnik, M.(2019) Sci Adv 5: eaaw3808-eaaw3808
- PubMed: 31328164
- DOI: https://doi.org/10.1126/sciadv.aaw3808
- Primary Citation of Related Structures:
6HXX, 6HXZ - PubMed Abstract:
Potato virus Y (PVY) is among the most economically important plant pathogens. Using cryoelectron microscopy, we determined the near-atomic structure of PVY's flexuous virions, revealing a previously unknown lumenal interplay between extended carboxyl-terminal regions of the coat protein units and viral RNA. RNA-coat protein interactions are crucial for the helical configuration and stability of the virion, as revealed by the unique near-atomic structure of RNA-free virus-like particles. The structures offer the first evidence for plasticity of the coat protein's amino- and carboxyl-terminal regions. Together with mutational analysis and in planta experiments, we show their crucial role in PVY infectivity and explain the ability of the coat protein to perform multiple biological tasks. Moreover, the high modularity of PVY virus-like particles suggests their potential as a new molecular scaffold for nanobiotechnological applications.
Organizational Affiliation:
Department of Molecular Biology and Nanobiotechnology, National Institute of Chemistry, Hajdrihova 19, 1000 Ljubljana, Slovenia.