Crystal structure and functional characterization of selenocysteine-containing glutathione peroxidase 4 suggests an alternative mechanism of peroxide reduction.
Borchert, A., Kalms, J., Roth, S.R., Rademacher, M., Schmidt, A., Holzhutter, H.G., Kuhn, H., Scheerer, P.(2018) Biochim Biophys Acta 1863: 1095-1107
- PubMed: 29883798
- DOI: https://doi.org/10.1016/j.bbalip.2018.06.006
- Primary Citation of Related Structures:
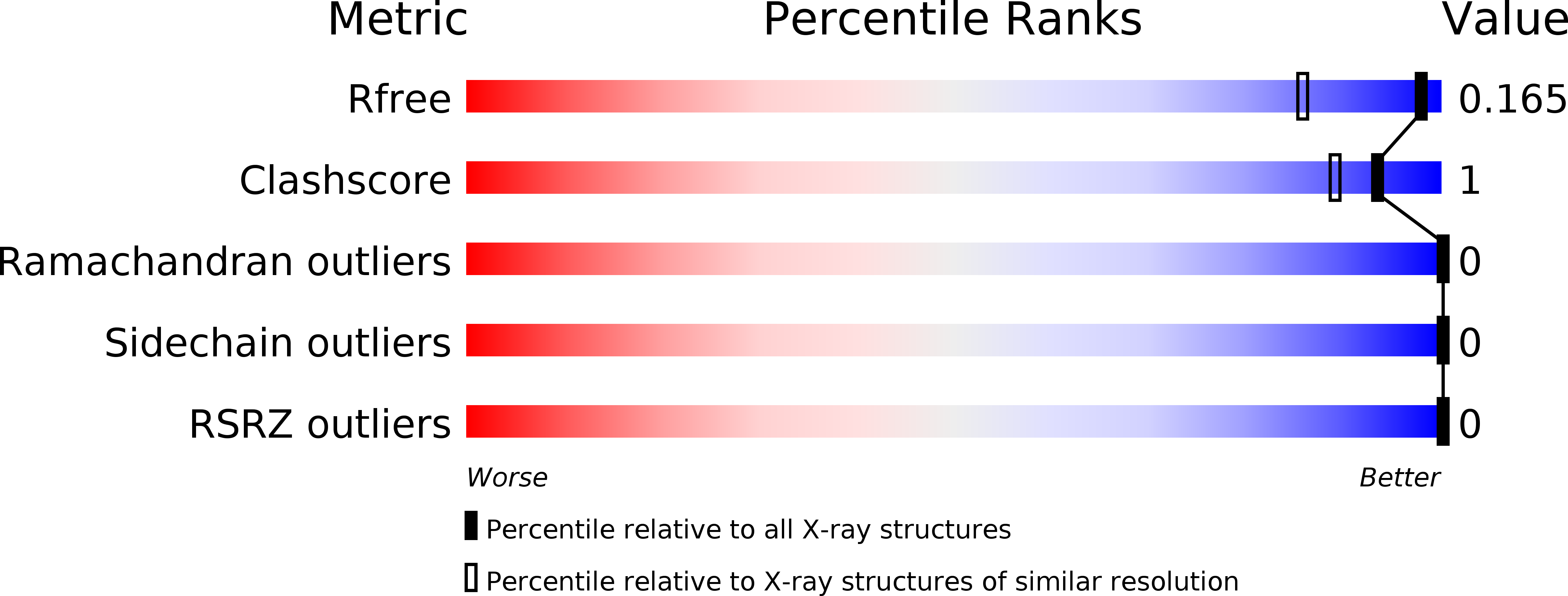
6ELW - PubMed Abstract:
Glutathione peroxidases (GPX) are anti-oxidative enzymes that reduce organic and inorganic hydroperoxides to the corresponding alcohols at the expense of reduced glutathione. The human genome involves eight GPX genes and five of them encode for selenocysteine-containing enzymes. Among the human GPX-isoforms, GPX4 is unique since it is capable of reducing complex hydroperoxy ester lipids such as hydroperoxy phospholipids and hydroperoxy cholesterolesters. Using a number of genetically modified mouse strains the biological role of GPX4 has comprehensively characterized but the molecular enzymology is less well explored. This lack of knowledge is partly related to the fact that mammalian selenoproteins are not high-level expressed in conventional overexpression systems. To explore the structural and functional properties of human GPX4 we expressed this selenoprotein in a cysteine-auxotrophic E. coli strain using a semi-chemical expression strategy. The recombinant enzyme was purified in mg amounts from the bacterial lysate to electrophoretic homogeneity and characterized with respect to its protein-chemical and enzymatic properties. Its crystal structure was solved at 1.3 Å resolution and the X-ray data indicated a monomeric protein, which contains the catalytic selenium at the redox level of the seleninic acid. These data suggest an alternative reaction mechanism involving three different redox states (selenol, selenenic acid, seleninic acid) of the catalytically active selenocysteine.
Organizational Affiliation:
Charité - Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Institut für Biochemie (CC2), Charitéplatz 1, D-10117 Berlin, Germany.