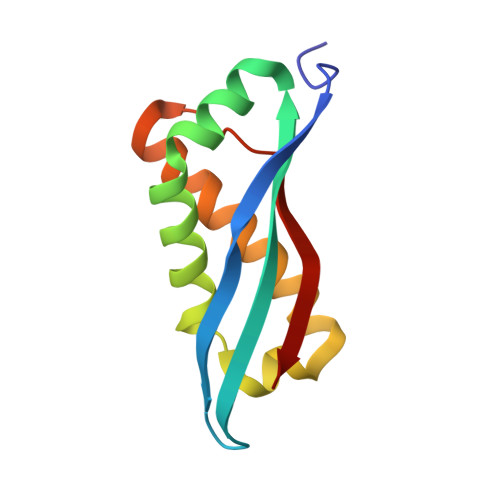
Structure ofVibrioFliL, a New Stomatin-like Protein That Assists the Bacterial Flagellar Motor Function.
Takekawa, N., Isumi, M., Terashima, H., Zhu, S., Nishino, Y., Sakuma, M., Kojima, S., Homma, M., Imada, K.(2019) mBio 10
- PubMed: 30890608
- DOI: https://doi.org/10.1128/mBio.00292-19
- Primary Citation of Related Structures:
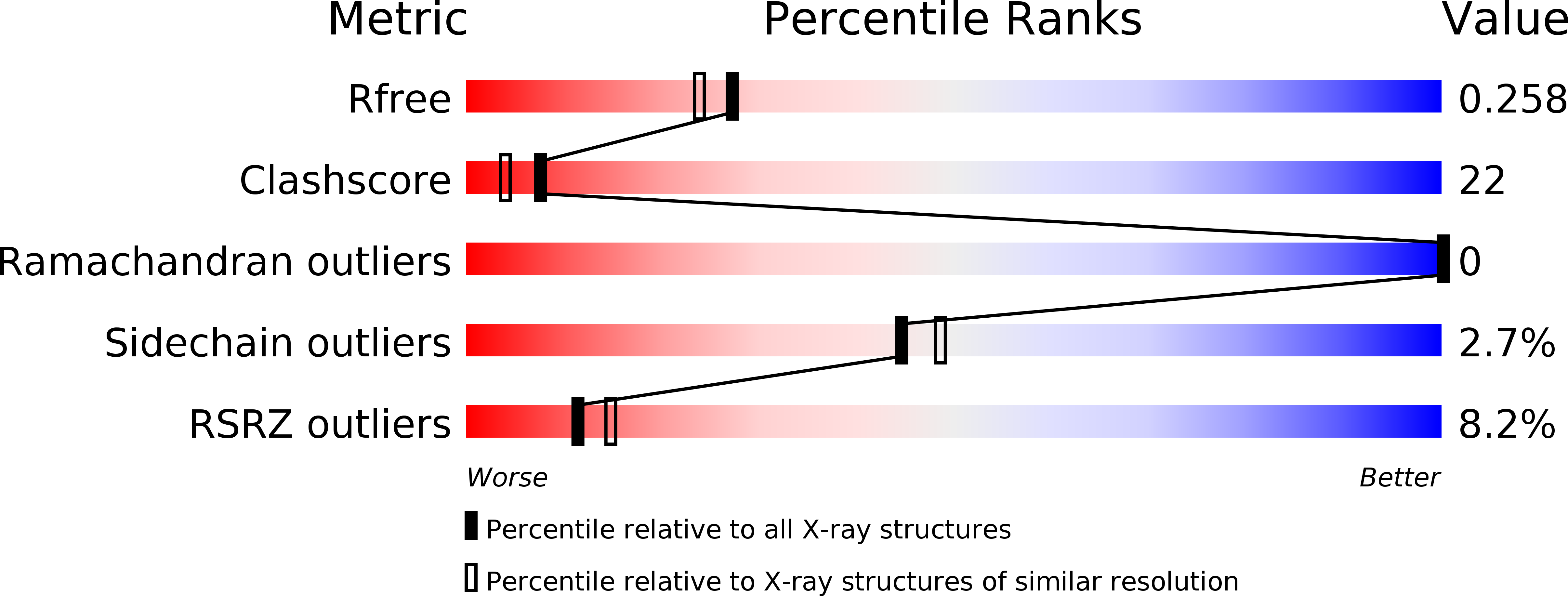
6AHP, 6AHQ - PubMed Abstract:
Many motile bacteria swim or swarm using a filamentous rotating organelle, the flagellum. FliL, a component protein of the flagellar motor, is known to enhance the motor performance under high-load conditions in some bacteria. Here we determined the structure of the periplasmic region of FliL (FliL Peri ) of the polar flagellum of Vibrio alginolyticus FliL Peri shows a remarkable structural similarity to the stomatin/prohibitin/flotillin/HflK/C (SPFH) domain of stomatin family proteins, some of which are involved in modulation of ion channel activities in various organisms. FliL Peri forms a ring assembly in the crystal with an inner diameter of around 8 nm, which is comparable to the size of the stator unit. Mutational analyses suggest that the FliL ring forms a complex with the stator unit and that the length of the periplasmic linkers of FliL and the stator B-subunit is essential for the complex formation. We propose a model of the FliL-stator complex to discuss how Vibrio FliL modulates stator function in the bacterial flagellar motor under conditions of high viscosity. IMPORTANCE Some flagellated bacteria regulate motor torque in response to the external load change. This behavior is critical for survival, but the mechanism has remained unknown. Here, we focused on a key protein, FliL of Vibrio alginolyticus , and solved the crystal structure of its periplasmic region (FliL Peri ). FliL Peri reveals striking structural similarity to a conserved domain of stomatin, which is involved in ion channel regulation in some organisms, including mammals. FliL Peri forms a ring with an inner diameter that is comparable in size to the stator unit. The mutational analyses suggested that the presence of the ring-like assembly of FliL around the stator unit enhances the surface swarming of Vibrio cells. Our study data also imply that the structural element for the ion channel regulation is conserved from bacteria to mammals.
Organizational Affiliation:
Department of Macromolecular Science, Graduate School of Science, Osaka University, Toyonaka, Osaka, Japan.