6OEL
Engineered Fab bound to IL-4 receptor
- PDB DOI: https://doi.org/10.2210/pdb6OEL/pdb
- Classification: IMMUNE SYSTEM
- Organism(s): Homo sapiens
- Expression System: Trichoplusia ni
- Mutation(s): Yes
- Deposited: 2019-03-27 Released: 2019-08-07
- Funding Organization(s): National Institutes of Health/National Institute Of Allergy and Infectious Diseases (NIH/NIAID)
Experimental Data Snapshot
- Method: X-RAY DIFFRACTION
- Resolution: 3.10 Å
- R-Value Free: 0.256
- R-Value Work: 0.199
- R-Value Observed: 0.202
wwPDB Validation 3D Report Full Report
Currently 6OEL does not have a validation slider image.
This is version 2.1 of the entry. See complete history.
Macromolecules
Find similar proteins by:
(by identity cutoff) | 3D Structure
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| engineered Interleukin-4, RGA variant | 129 | Homo sapiens | Mutation(s): 8 Gene Names: IL4 | 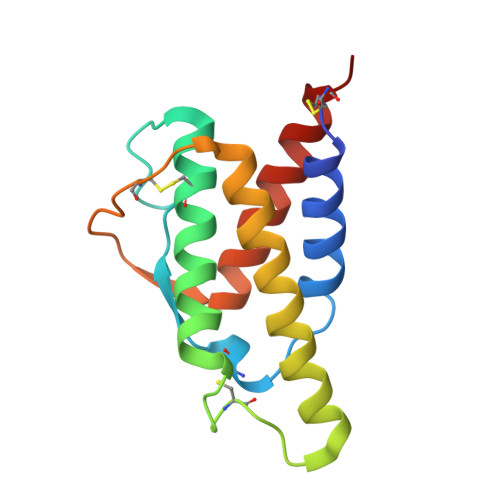 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P05112 (Homo sapiens) Explore P05112 Go to UniProtKB: P05112 | |||||
PHAROS: P05112 GTEx: ENSG00000113520 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P05112 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by:
(by identity cutoff) | 3D Structure
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Interleukin-4 receptor subunit alpha | 202 | Homo sapiens | Mutation(s): 0 Gene Names: IL4R, IL4RA, 582J2.1 | 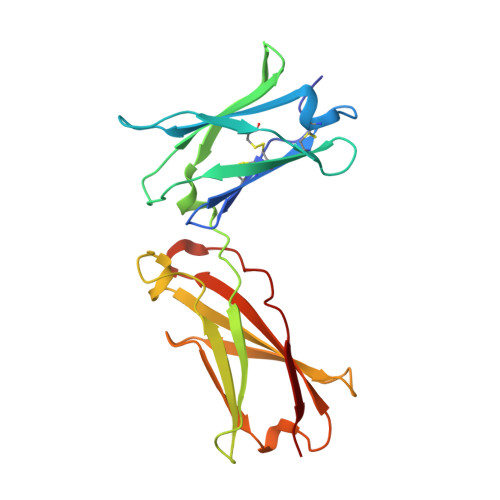 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P24394 (Homo sapiens) Explore P24394 Go to UniProtKB: P24394 | |||||
PHAROS: P24394 GTEx: ENSG00000077238 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P24394 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by:
(by identity cutoff) | 3D Structure
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Cytokine receptor common subunit gamma | 189 | Homo sapiens | Mutation(s): 1 Gene Names: IL2RG | 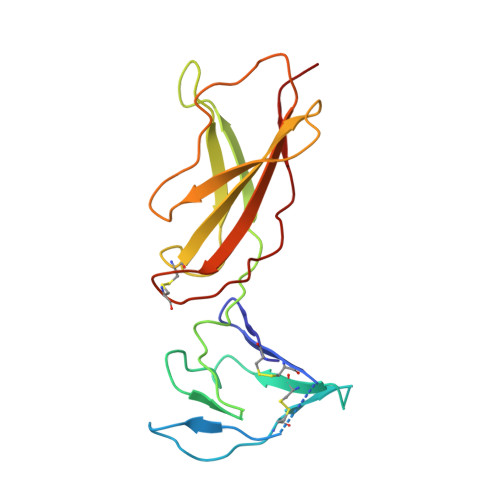 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P31785 (Homo sapiens) Explore P31785 Go to UniProtKB: P31785 | |||||
PHAROS: P31785 GTEx: ENSG00000147168 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P31785 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by:
(by identity cutoff) | 3D Structure
Entity ID: 4 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| engineered Fab heavy chain | D [auth H] | 229 | Homo sapiens | Mutation(s): 0 | 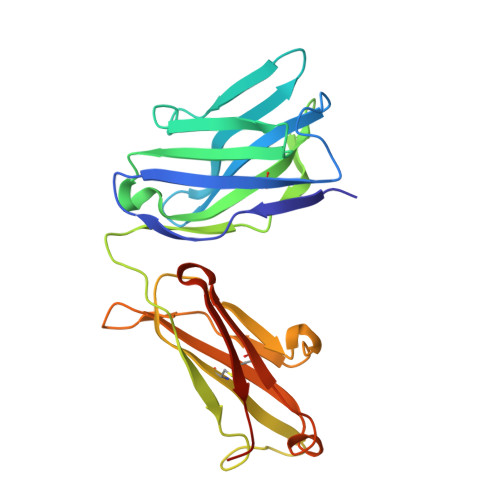 |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by:
(by identity cutoff) | 3D Structure
Entity ID: 5 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| engineered Fab light chain | E [auth L] | 224 | Homo sapiens | Mutation(s): 0 |  |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
Oligosaccharides
Small Molecules
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| NAG Query on NAG | G [auth B], H [auth B], I [auth B], J [auth B], K [auth C] | 2-acetamido-2-deoxy-beta-D-glucopyranose C8 H15 N O6 OVRNDRQMDRJTHS-FMDGEEDCSA-N |  | ||
| NO3 Query on NO3 | L [auth H] | NITRATE ION N O3 NHNBFGGVMKEFGY-UHFFFAOYSA-N |  | ||
Experimental Data & Validation
Experimental Data
- Method: X-RAY DIFFRACTION
- Resolution: 3.10 Å
- R-Value Free: 0.256
- R-Value Work: 0.199
- R-Value Observed: 0.202
- Space Group: F 41 3 2
- Diffraction Data: https://doi.org/10.15785/SBGRID/652
Unit Cell:
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 328.1 | α = 90 |
| b = 328.1 | β = 90 |
| c = 328.1 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| XDS | data reduction |
| XDS | data scaling |
| PHASER | phasing |
Structure Validation
Currently 6OEL does not have a validation slider image.
Entry History & Funding Information
Deposition Data
- Released Date: 2019-08-07 Deposition Author(s): Jude, K.M., Moraga, I., Spangler, J.B., Garcia, K.C.
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Institutes of Health/National Institute Of Allergy and Infectious Diseases (NIH/NIAID) | United States | R01 AI51321 |
Revision History (Full details and data files)
- Version 1.0: 2019-08-07
Type: Initial release - Version 1.1: 2019-08-21
Changes: Data collection, Database references - Version 1.2: 2019-10-02
Changes: Data collection, Database references - Version 1.3: 2019-12-18
Changes: Author supporting evidence - Version 2.0: 2020-07-29
Type: Remediation
Reason: Carbohydrate remediation
Changes: Atomic model, Data collection, Derived calculations, Structure summary - Version 2.1: 2023-10-11
Changes: Data collection, Database references, Derived calculations, Refinement description, Structure summary













