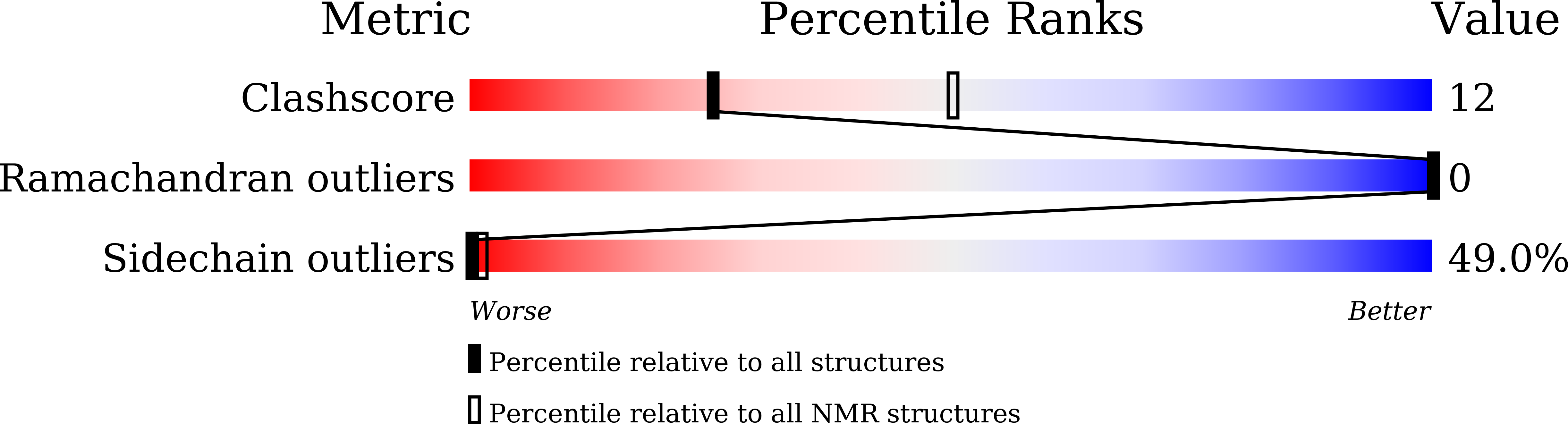Structural basis for endotoxin neutralisation and anti-inflammatory activity of thrombin-derived C-terminal peptides.
Saravanan, R., Holdbrook, D.A., Petrlova, J., Singh, S., Berglund, N.A., Choong, Y.K., Kjellstrom, S., Bond, P.J., Malmsten, M., Schmidtchen, A.(2018) Nat Commun 9: 2762-2762
- PubMed: 30018388
- DOI: https://doi.org/10.1038/s41467-018-05242-0
- Primary Citation of Related Structures:
5Z5W, 5Z5X - PubMed Abstract:
Thrombin-derived C-terminal peptides (TCPs) of about 2 kDa are present in wounds, where they exert anti-endotoxic functions. Employing a combination of nuclear magnetic resonance spectroscopy (NMR), biophysical, mass spectrometry and cellular studies combined with in silico multiscale modelling, we here determine the bound conformation of HVF18 (HVFRLKKWIQKVIDQFGE), a TCP generated by neutrophil elastase, in complex with bacterial lipopolysaccharide (LPS) and define a previously undisclosed interaction between TCPs and human CD14. Further, we show that TCPs bind to the LPS-binding hydrophobic pocket of CD14 and identify the peptide region crucial for TCP interaction with LPS and CD14. Taken together, our results demonstrate the role of structural transitions in LPS complex formation and CD14 interaction, providing a molecular explanation for the previously observed therapeutic effects of TCPs in experimental models of bacterial sepsis and endotoxin shock.
Organizational Affiliation:
Lee Kong Chian School of Medicine, Nanyang Technological University, 59 Nanyang Drive, Singapore, 636921, Singapore. rathi@ntu.edu.sg.