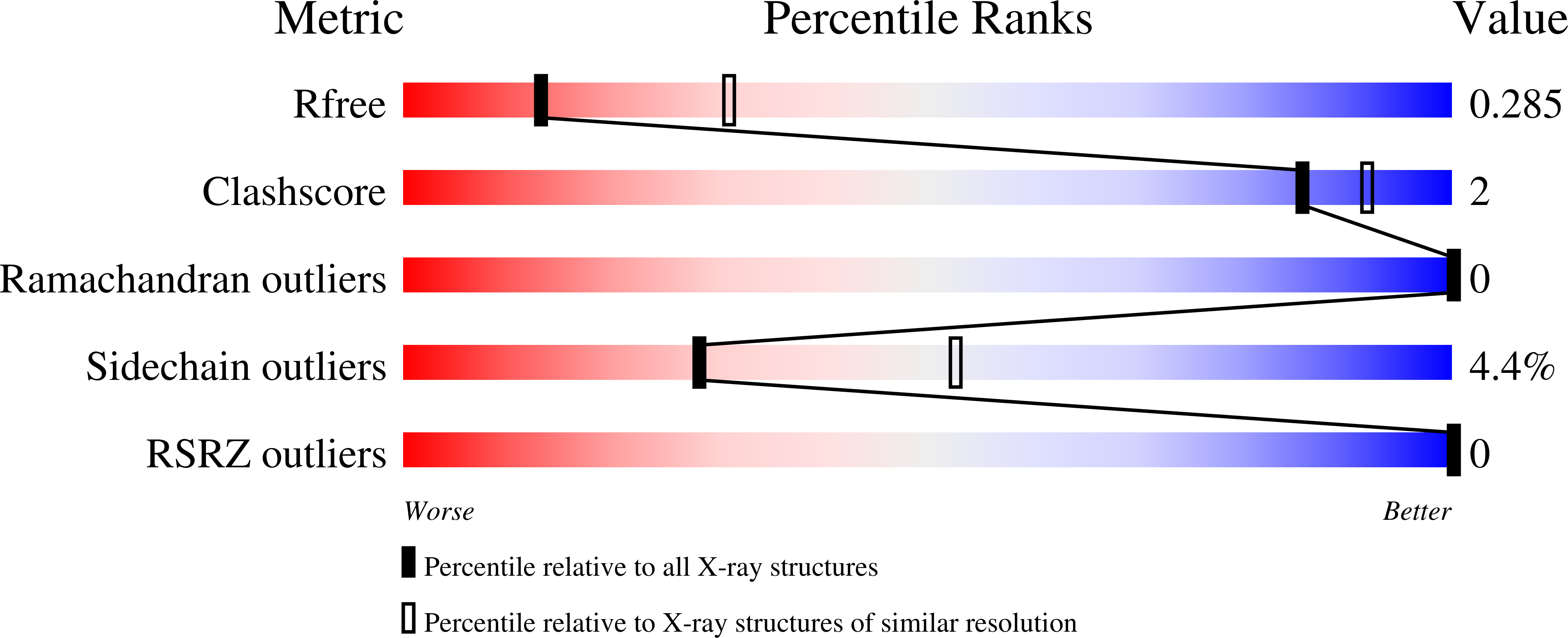
Activator Protein-1: redox switch controlling structure and DNA-binding.
Yin, Z., Machius, M., Nestler, E.J., Rudenko, G.(2017) Nucleic Acids Res 45: 11425-11436
- PubMed: 28981703
- DOI: https://doi.org/10.1093/nar/gkx795
- Primary Citation of Related Structures:
5VPA, 5VPB, 5VPC, 5VPD, 5VPE, 5VPF - PubMed Abstract:
The transcription factor, activator protein-1 (AP-1), binds to cognate DNA under redox control; yet, the underlying mechanism has remained enigmatic. A series of crystal structures of the AP-1 FosB/JunD bZIP domains reveal ordered DNA-binding regions in both FosB and JunD even in absence DNA. However, while JunD is competent to bind DNA, the FosB bZIP domain must undergo a large conformational rearrangement that is controlled by a 'redox switch' centered on an inter-molecular disulfide bond. Solution studies confirm that FosB/JunD cannot undergo structural transition and bind DNA when the redox-switch is in the 'OFF' state, and show that the mid-point redox potential of the redox switch affords it sensitivity to cellular redox homeostasis. The molecular and structural studies presented here thus reveal the mechanism underlying redox-regulation of AP-1 Fos/Jun transcription factors and provide structural insight for therapeutic interventions targeting AP-1 proteins.
Organizational Affiliation:
Department of Pharmacology and Toxicology, and the Sealy Center for Structural Biology, University of Texas Medical Branch, Galveston, TX 77555, USA.