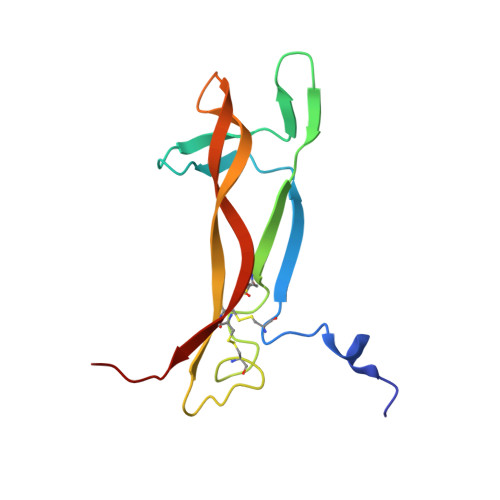
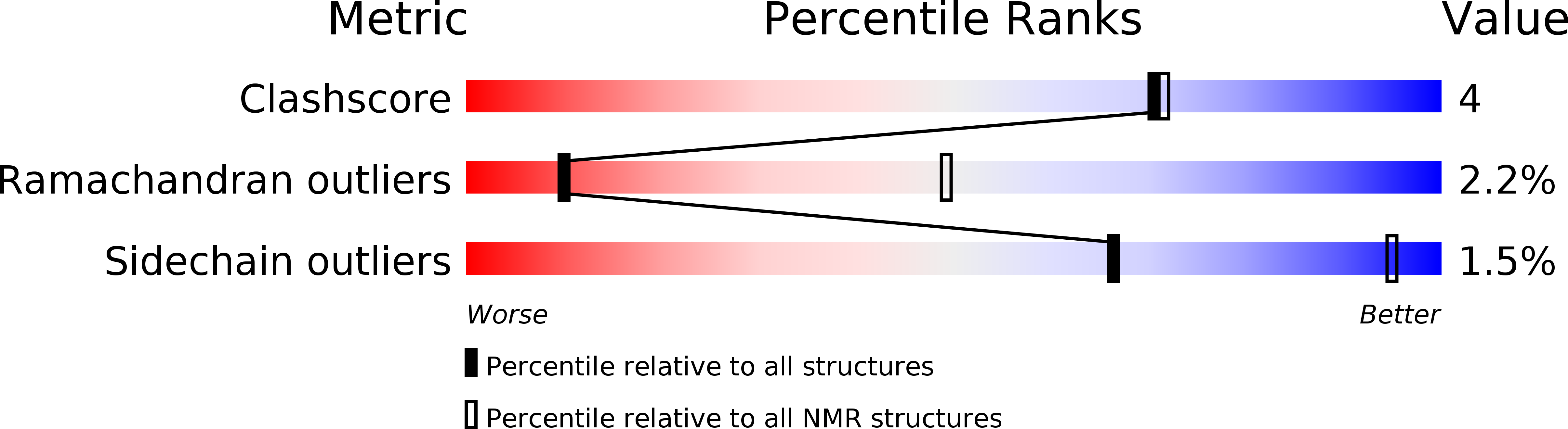Conformational Rigidity within Plasticity Promotes Differential Target Recognition of Nerve Growth Factor.
Paoletti, F., de Chiara, C., Kelly, G., Covaceuszach, S., Malerba, F., Yan, R., Lamba, D., Cattaneo, A., Pastore, A.(2016) Front Mol Biosci 3: 83-83
- PubMed: 28083536
- DOI: https://doi.org/10.3389/fmolb.2016.00083
- Primary Citation of Related Structures:
5LSD - PubMed Abstract:
Nerve Growth Factor (NGF), the prototype of the neurotrophin family, is essential for maintenance and growth of different neuronal populations. The X-ray crystal structure of NGF has been known since the early '90s and shows a β-sandwich fold with extensive loops that are involved in the interaction with its binding partners. Understanding the dynamical properties of these loops is thus important for molecular recognition. We present here a combined solution NMR/molecular dynamics study which addresses the question of whether and how much the long loops of NGF are flexible and describes the N-terminal intrinsic conformational tendency of the unbound NGF molecule. NMR titration experiments allowed identification of a previously undetected epitope of the anti-NGF antagonist antibody αD11 which will be of crucial importance for future drug lead discovery. The present study thus recapitulates all the available structural information and unveils the conformational versatility of the relatively rigid NGF loops upon functional ligand binding.
Organizational Affiliation:
Neurotrophic Factors and Neurodegenerative Diseases Unit, European Brain Research, Rita Levi-Montalcini FoundationRome, Italy; Scuola Normale SuperiorePisa, Italy.