Listeria monocytogenes InlP interacts with afadin and facilitates basement membrane crossing.
Faralla, C., Bastounis, E.E., Ortega, F.E., Light, S.H., Rizzuto, G., Gao, L., Marciano, D.K., Nocadello, S., Anderson, W.F., Robbins, J.R., Theriot, J.A., Bakardjiev, A.I.(2018) PLoS Pathog 14: e1007094-e1007094
- PubMed: 29847585
- DOI: https://doi.org/10.1371/journal.ppat.1007094
- Primary Citation of Related Structures:
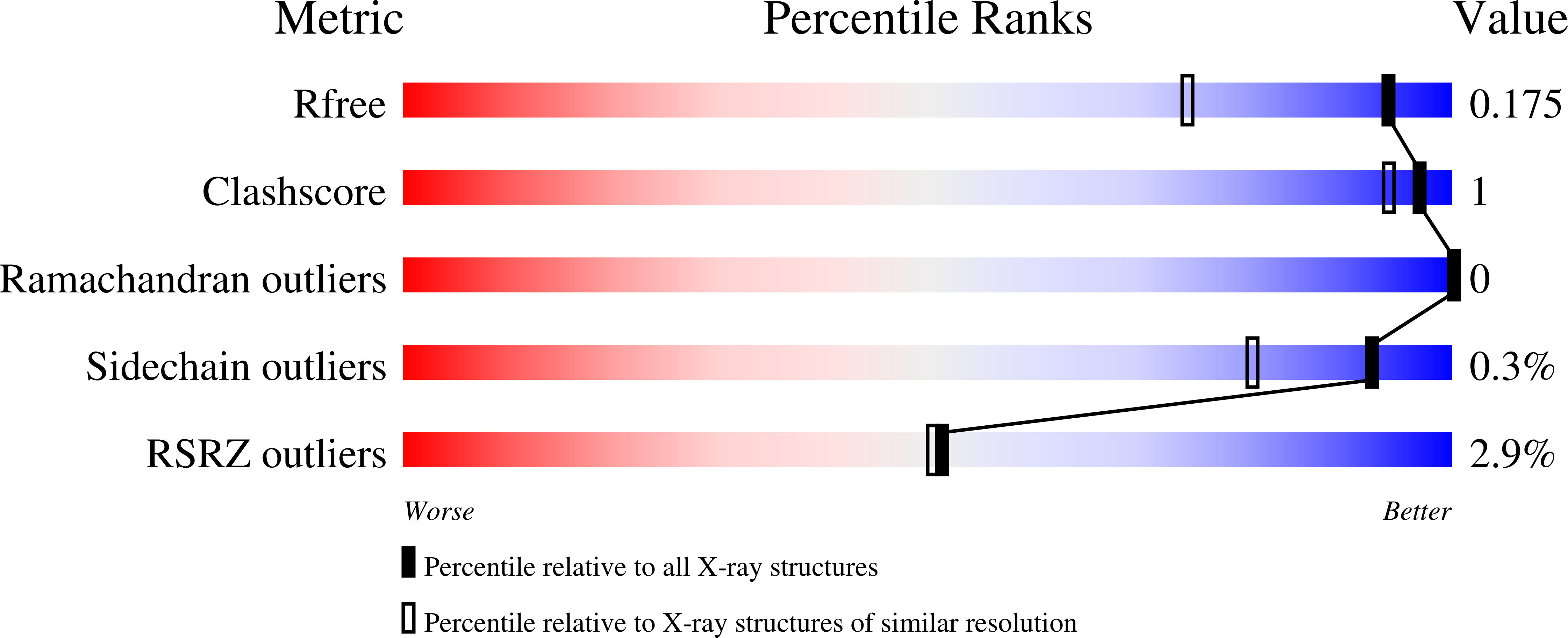
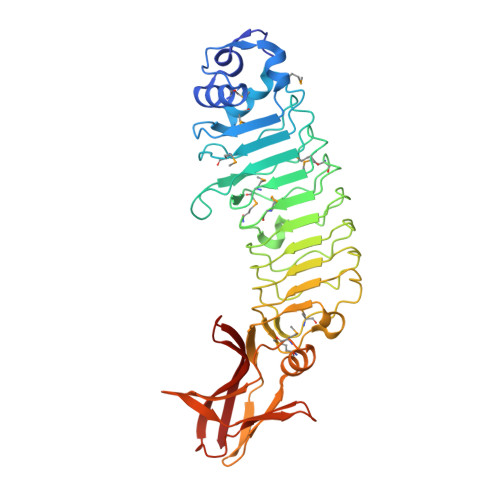
5HL3, 5KZS - PubMed Abstract:
During pregnancy, the placenta protects the fetus against the maternal immune response, as well as bacterial and viral pathogens. Bacterial pathogens that have evolved specific mechanisms of breaching this barrier, such as Listeria monocytogenes, present a unique opportunity for learning how the placenta carries out its protective function. We previously identified the L. monocytogenes protein Internalin P (InlP) as a secreted virulence factor critical for placental infection. Here, we show that InlP, but not the highly similar L. monocytogenes internalin Lmo2027, binds to human afadin (encoded by AF-6), a protein associated with cell-cell junctions. A crystal structure of InlP reveals several unique features, including an extended leucine-rich repeat (LRR) domain with a distinctive Ca2+-binding site. Despite afadin's involvement in the formation of cell-cell junctions, MDCK epithelial cells expressing InlP displayed a decrease in the magnitude of the traction stresses they could exert on deformable substrates, similar to the decrease in traction exhibited by AF-6 knock-out MDCK cells. L. monocytogenes ΔinlP mutants were deficient in their ability to form actin-rich protrusions from the basal face of polarized epithelial monolayers, a necessary step in the crossing of such monolayers (transcytosis). A similar phenotype was observed for bacteria expressing an internal in-frame deletion in inlP (inlP ΔLRR5) that specifically disrupts its interaction with afadin. However, afadin deletion in the host cells did not rescue the transcytosis defect. We conclude that secreted InlP targets cytosolic afadin to specifically promote L. monocytogenes transcytosis across the basal face of epithelial monolayers, which may contribute to the crossing of the basement membrane during placental infection.
Organizational Affiliation:
Benioff Children's Hospital, University of California, San Francisco, San Francisco, California, United States of America.