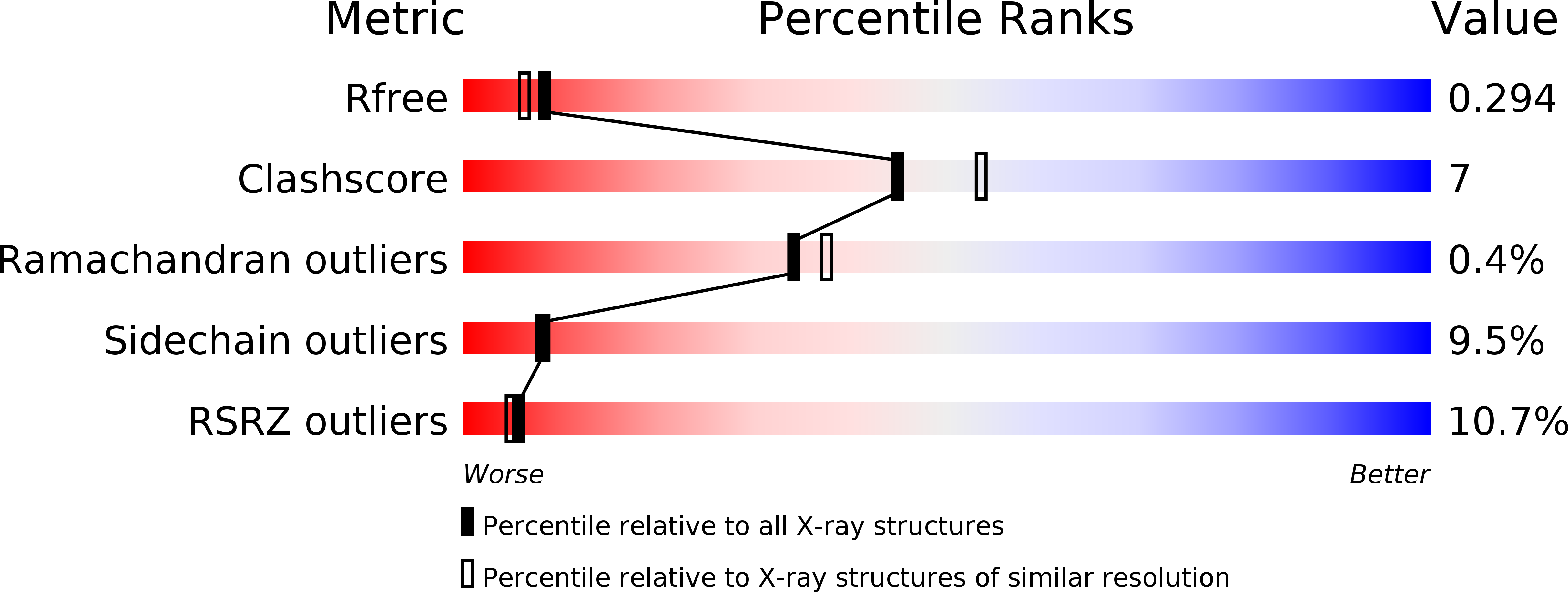
Two distinct DNA sequences recognized by transcription factors represent enthalpy and entropy optima.
Morgunova, E., Yin, Y., Das, P.K., Jolma, A., Zhu, F., Popov, A., Xu, Y., Nilsson, L., Taipale, J.(2018) Elife 7
- PubMed: 29638214
- DOI: https://doi.org/10.7554/eLife.32963
- Primary Citation of Related Structures:
5EDN, 5EEA, 6ES2, 6ES3 - PubMed Abstract:
Most transcription factors (TFs) can bind to a population of sequences closely related to a single optimal site. However, some TFs can bind to two distinct sequences that represent two local optima in the Gibbs free energy of binding (ΔG). To determine the molecular mechanism behind this effect, we solved the structures of human HOXB13 and CDX2 bound to their two optimal DNA sequences, CAATAAA and TCGTAAA. Thermodynamic analyses by isothermal titration calorimetry revealed that both sites were bound with similar ΔG. However, the interaction with the CAA sequence was driven by change in enthalpy (ΔH), whereas the TCG site was bound with similar affinity due to smaller loss of entropy (ΔS). This thermodynamic mechanism that leads to at least two local optima likely affects many macromolecular interactions, as ΔG depends on two partially independent variables ΔH and ΔS according to the central equation of thermodynamics, ΔG = ΔH - TΔS.
Organizational Affiliation:
Department of Medical Biochemistry and Biophysics, Karolinska Institutet, Stockholm, Sweden.