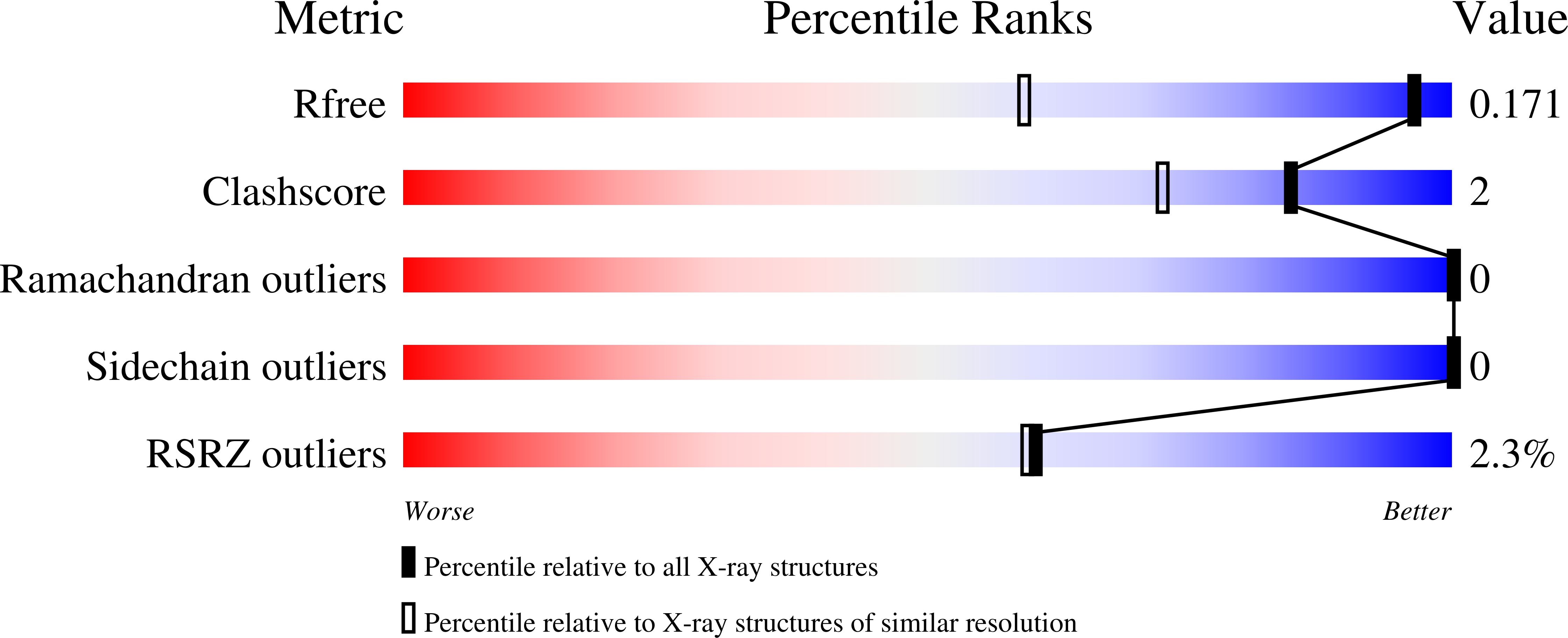
Structure-based design and synthesis of triazole-based macrocyclic inhibitors of norovirus protease: Structural, biochemical, spectroscopic, and antiviral studies.
Weerawarna, P.M., Kim, Y., Galasiti Kankanamalage, A.C., Damalanka, V.C., Lushington, G.H., Alliston, K.R., Mehzabeen, N., Battaile, K.P., Lovell, S., Chang, K.O., Groutas, W.C.(2016) Eur J Med Chem 119: 300-318
- PubMed: 27235842
- DOI: https://doi.org/10.1016/j.ejmech.2016.04.013
- Primary Citation of Related Structures:
5E0G, 5E0H, 5E0J - PubMed Abstract:
Outbreaks of acute gastroenteritis caused by noroviruses constitute a public health concern worldwide. To date, there are no approved drugs or vaccines for the management and prophylaxis of norovirus infections. A potentially effective strategy for the development of norovirus therapeutics entails the discovery of inhibitors of norovirus 3CL protease, an enzyme essential for noroviral replication. We describe herein the structure-based design of the first class of permeable, triazole-based macrocyclic inhibitors of norovirus 3C-like protease, as well as pertinent X-ray crystallographic, biochemical, spectroscopic, and antiviral studies.
Organizational Affiliation:
Department of Chemistry, Wichita State University, Wichita, KS 67260, USA.