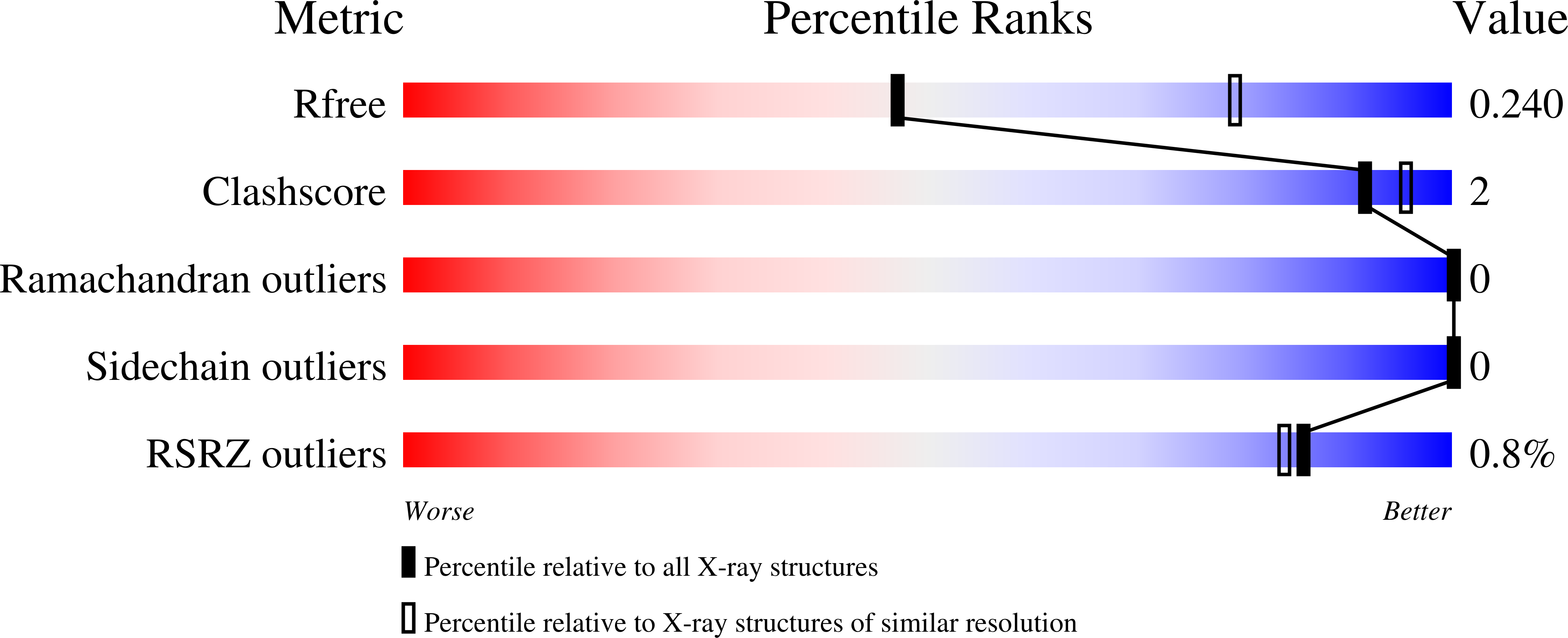
Characterization of novel small-molecule NRF2 activators: Structural and biochemical validation of stereospecific KEAP1 binding.
Huerta, C., Jiang, X., Trevino, I., Bender, C.F., Ferguson, D.A., Probst, B., Swinger, K.K., Stoll, V.S., Thomas, P.J., Dulubova, I., Visnick, M., Wigley, W.C.(2016) Biochim Biophys Acta 1860: 2537-2552
- PubMed: 27474998
- DOI: https://doi.org/10.1016/j.bbagen.2016.07.026
- Primary Citation of Related Structures:
5DAD, 5DAF - PubMed Abstract:
Semi-synthetic oleanane triterpenoid antioxidant inflammation modulators (tpAIMs) are small molecules that interact with KEAP1 cysteine residue 151 (C151) and activate NRF2. Exploration of the structure-activity relationship between the tpAIMs and KEAP1 is limited by the predominantly hydrocarbon nature of the oleanane triterpenoid pentacyclic ring structure. Therefore, we used novel, chemically-tractable, synthetic antioxidant inflammation modulators (sAIMs) to probe the stereoselectivity of the ligand-protein interaction.
Organizational Affiliation:
Department of Research, Reata Pharmaceuticals, Inc., Irving, TX 75063, United States; Department of Physiology, UT Southwestern Medical Center, Dallas, TX 75390, United States.