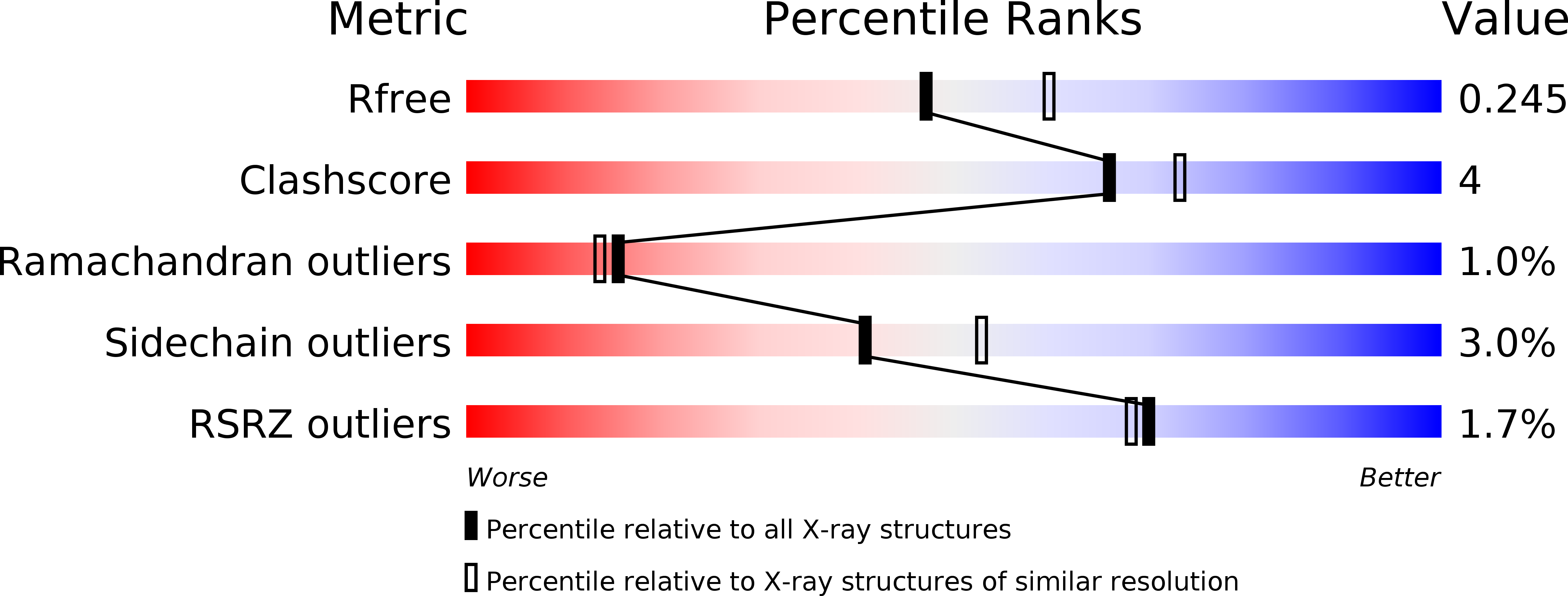
Antagonism screen for inhibitors of bacterial cell wall biogenesis uncovers an inhibitor of undecaprenyl diphosphate synthase.
Farha, M.A., Czarny, T.L., Myers, C.L., Worrall, L.J., French, S., Conrady, D.G., Wang, Y., Oldfield, E., Strynadka, N.C., Brown, E.D.(2015) Proc Natl Acad Sci U S A 112: 11048-11053
- PubMed: 26283394
- DOI: https://doi.org/10.1073/pnas.1511751112
- Primary Citation of Related Structures:
5CQB, 5CQJ - PubMed Abstract:
Drug combinations are valuable tools for studying biological systems. Although much attention has been given to synergistic interactions in revealing connections between cellular processes, antagonistic interactions can also have tremendous value in elucidating genetic networks and mechanisms of drug action. Here, we exploit the power of antagonism in a high-throughput screen for molecules that suppress the activity of targocil, an inhibitor of the wall teichoic acid (WTA) flippase in Staphylococcus aureus. Well-characterized antagonism within the WTA biosynthetic pathway indicated that early steps would be sensitive to this screen; however, broader interactions with cell wall biogenesis components suggested that it might capture additional targets. A chemical screening effort using this approach identified clomiphene, a widely used fertility drug, as one such compound. Mechanistic characterization revealed the target was the undecaprenyl diphosphate synthase, an enzyme that catalyzes the synthesis of a polyisoprenoid essential for both peptidoglycan and WTA synthesis. The work sheds light on mechanisms contributing to the observed suppressive interactions of clomiphene and in turn reveals aspects of the biology that underlie cell wall synthesis in S. aureus. Further, this effort highlights the utility of antagonistic interactions both in high-throughput screening and in compound mode of action studies. Importantly, clomiphene represents a lead for antibacterial drug discovery.
Organizational Affiliation:
Department of Biochemistry and Biomedical Sciences, McMaster University, Hamilton, ON, Canada L8N 3Z5; Michael G. DeGroote Institute of Infectious Disease Research, McMaster University, Hamilton, ON, Canada L8N 3Z5;