ROCK inhibitors 2. Improving potency, selectivity and solubility through the application of rationally designed solubilizing groups.
Gao, H., Marhefka, C., Jacobs, M.D., Cao, J., Bandarage, U.K., Green, J.(2018) Bioorg Med Chem Lett
- PubMed: 29945794
- DOI: https://doi.org/10.1016/j.bmcl.2018.06.043
- Primary Citation of Related Structures:
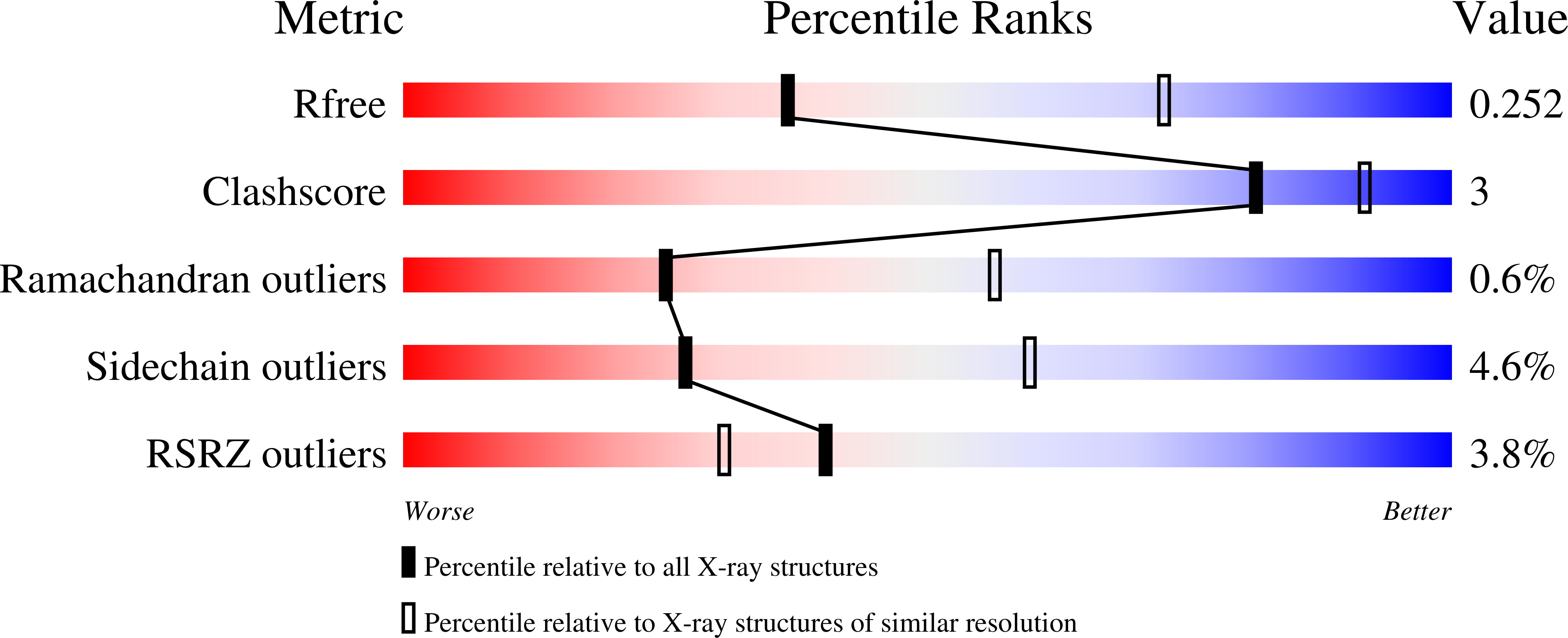
5HVU - PubMed Abstract:
Solubilizing groups have been frequently appended to kinase inhibitor drug molecules when solubility is insufficient for pharmaceutical development. Such groups are usually located at substitution sites that have minimal impact on target activity. In this report we describe the incorporation of solubilizing groups in a class of Rho kinase (ROCK) inhibitors that not only confer improved solubility, but also enhance target potency and selectivity against a closely related kinase, PKA.
Organizational Affiliation:
Vertex Pharmaceuticals, Incorporated, 50 Northern Avenue, Boston, MA 02210, USA.