Crystal Structure of PKG I:cGMP Complex Reveals a cGMP-Mediated Dimeric Interface that Facilitates cGMP-Induced Activation.
Kim, J.J., Lorenz, R., Arold, S.T., Reger, A.S., Sankaran, B., Casteel, D.E., Herberg, F.W., Kim, C.(2016) Structure 24: 710-720
- PubMed: 27066748
- DOI: https://doi.org/10.1016/j.str.2016.03.009
- Primary Citation of Related Structures:
4Z07 - PubMed Abstract:
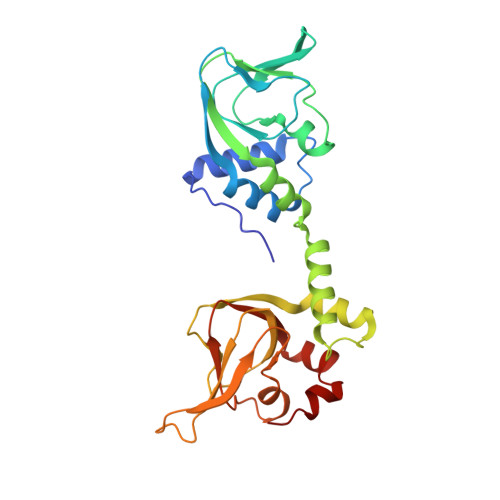
Cyclic guanosine monophosphate (cGMP)-dependent protein kinase (PKG) is a key regulator of smooth muscle and vascular tone and represents an important drug target for treating hypertensive diseases and erectile dysfunction. Despite its importance, its activation mechanism is not fully understood. To understand the activation mechanism, we determined a 2.5 Å crystal structure of the PKG I regulatory (R) domain bound with cGMP, which represents the activated state. Although we used a monomeric domain for crystallization, the structure reveals that two R domains form a symmetric dimer where the cGMP bound at high-affinity pockets provide critical dimeric contacts. Small-angle X-ray scattering and mutagenesis support this dimer model, suggesting that the dimer interface modulates kinase activation. Finally, structural comparison with the homologous cyclic AMP-dependent protein kinase reveals that PKG is drastically different from protein kinase A in its active conformation, suggesting a novel activation mechanism for PKG.
Organizational Affiliation:
Department of Pharmacology, Baylor College of Medicine, Houston, TX 77030, USA; Department of Biochemistry, University of Kassel, Kassel, Hesse 34132, Germany.