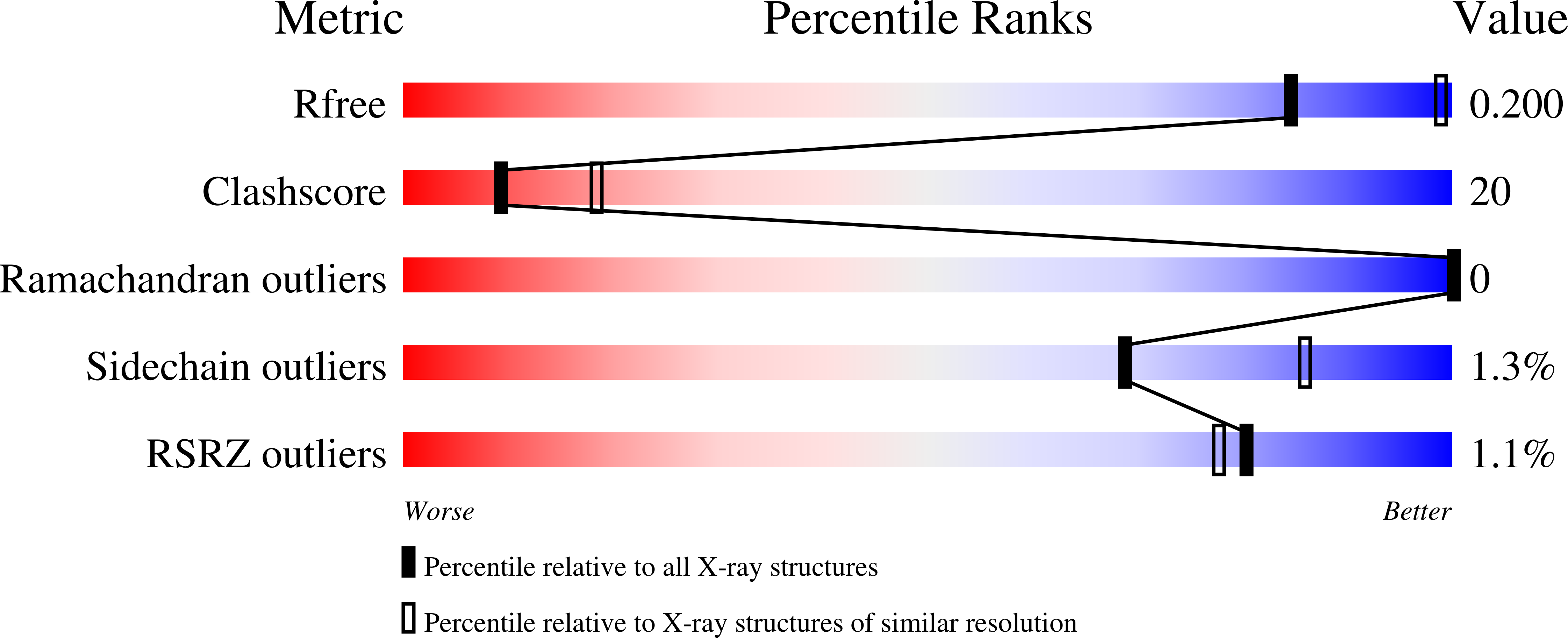
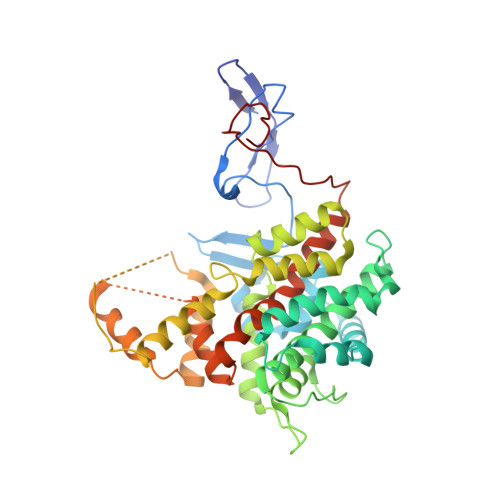
Structures of citrate synthase and malate dehydrogenase of Mycobacterium tuberculosis.
Ferraris, D.M., Spallek, R., Oehlmann, W., Singh, M., Rizzi, M.(2015) Proteins 83: 389-394
- PubMed: 25524525
- DOI: https://doi.org/10.1002/prot.24743
- Primary Citation of Related Structures:
4TVM, 4TVO - PubMed Abstract:
The tricarboxylic acid (TCA) cycle is a central metabolic pathway of all aerobic organisms and is responsible for the synthesis of many important precursors and molecules. TCA cycle plays a key role in the metabolism of Mycobacterium tuberculosis and is involved in the adaptation process of the bacteria to the host immune response. We present here the first crystal structures of M. tuberculosis malate dehydrogenase and citrate synthase, two consecutive enzymes of the TCA, at 2.6 Å and 1.5 Å resolution, respectively. General analogies and local differences with the previously reported homologous protein structures are described.
Organizational Affiliation:
Department of Pharmaceutical Sciences, Università del Piemonte Orientale "A. Avogadro", Largo Donegani 2, 28100, Novara, Italy.