The octahaem MccA is a haem c-copper sulfite reductase.
Hermann, B., Kern, M., La Pietra, L., Simon, J., Einsle, O.(2015) Nature 520: 706-709
- PubMed: 25642962
- DOI: https://doi.org/10.1038/nature14109
- Primary Citation of Related Structures:
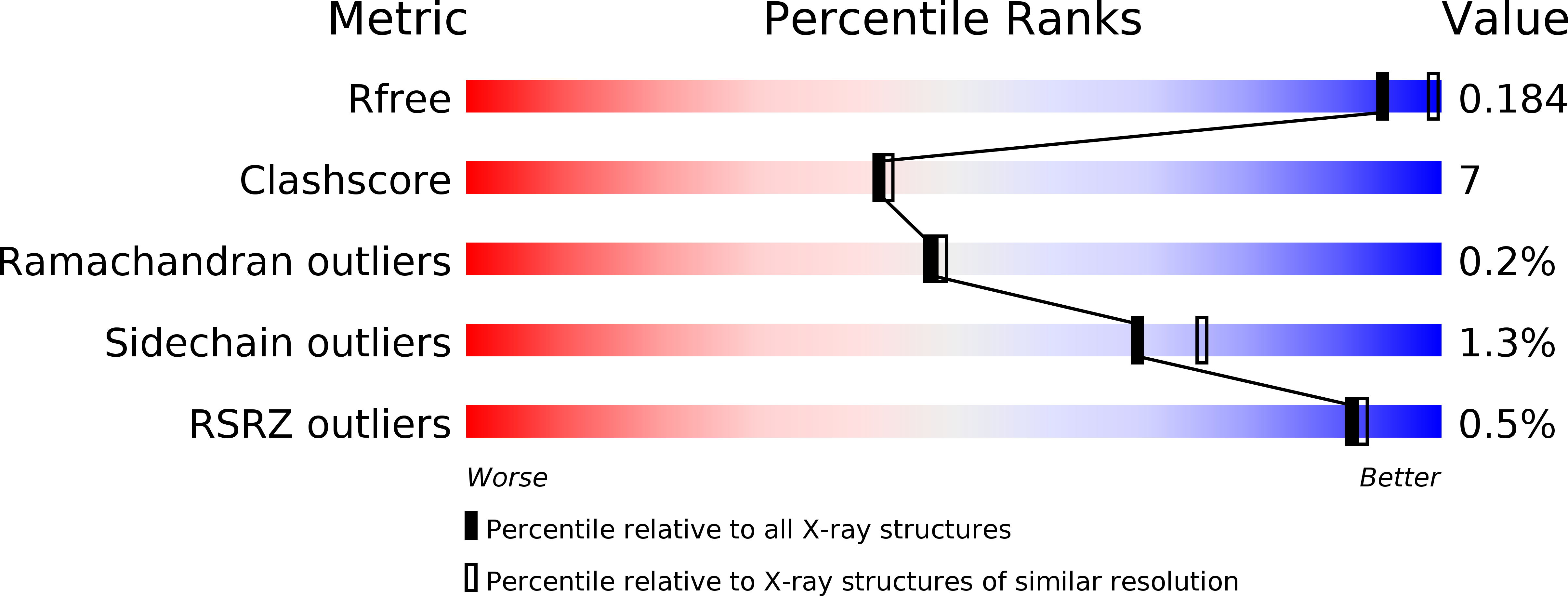
4RKM, 4RKN - PubMed Abstract:
The six-electron reduction of sulfite to sulfide is the pivot point of the biogeochemical cycle of the element sulfur. The octahaem cytochrome c MccA (also known as SirA) catalyses this reaction for dissimilatory sulfite utilization by various bacteria. It is distinct from known sulfite reductases because it has a substantially higher catalytic activity and a relatively low reactivity towards nitrite. The mechanistic reasons for the increased efficiency of MccA remain to be elucidated. Here we show that anoxically purified MccA exhibited a 2- to 5.5-fold higher specific sulfite reductase activity than the enzyme isolated under oxic conditions. We determined the three-dimensional structure of MccA to 2.2 Å resolution by single-wavelength anomalous dispersion. We find a homotrimer with an unprecedented fold and haem arrangement, as well as a haem bound to a CX15CH motif. The heterobimetallic active-site haem 2 has a Cu(I) ion juxtaposed to a haem c at a Fe-Cu distance of 4.4 Å. While the combination of metals is reminiscent of respiratory haem-copper oxidases, the oxidation-labile Cu(I) centre of MccA did not seem to undergo a redox transition during catalysis. Intact MccA tightly bound SO2 at haem 2, a dehydration product of the substrate sulfite that was partially turned over due to photoreduction by X-ray irradiation, yielding the reaction intermediate SO. Our data show the biometal copper in a new context and function and provide a chemical rationale for the comparatively high catalytic activity of MccA.
Organizational Affiliation:
Lehrstuhl Biochemie, Institut für Biochemie, Albert-Ludwigs-Universität Freiburg, Albertstrasse 21, 79104 Freiburg, Germany.