Molecular mechanisms of inhibition of influenza by surfactant protein d revealed by large-scale molecular dynamics simulation.
Goh, B.C., Rynkiewicz, M.J., Cafarella, T.R., White, M.R., Hartshorn, K.L., Allen, K., Crouch, E.C., Calin, O., Seeberger, P.H., Schulten, K., Seaton, B.A.(2013) Biochemistry 52: 8527-8538
- PubMed: 24224757
- DOI: https://doi.org/10.1021/bi4010683
- Primary Citation of Related Structures:
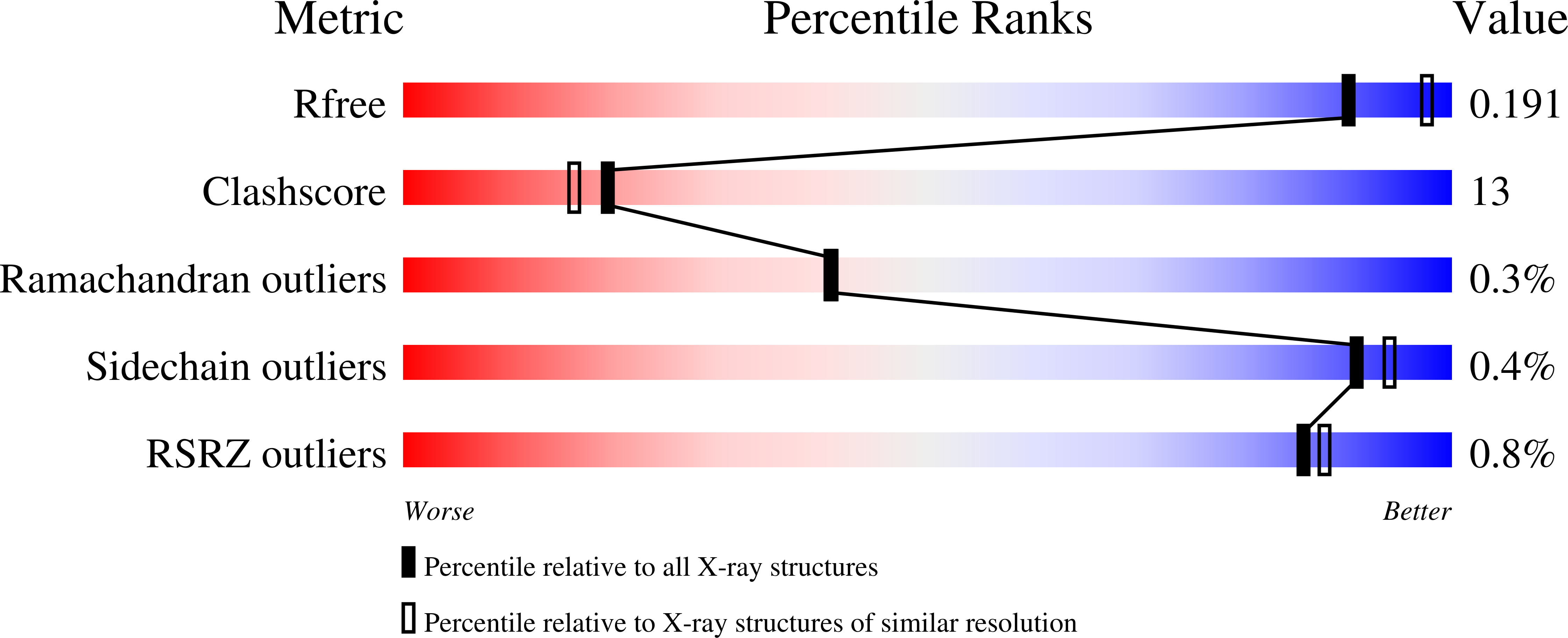
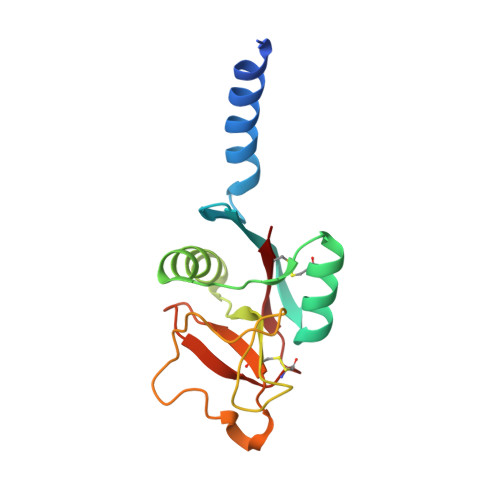
4M17, 4M18 - PubMed Abstract:
Surfactant protein D (SP-D), a mammalian C-type lectin, is the primary innate inhibitor of influenza A virus (IAV) in the lung. Interactions of SP-D with highly branched viral N-linked glycans on hemagglutinin (HA), an abundant IAV envelope protein and critical virulence factor, promote viral aggregation and neutralization through as yet unknown molecular mechanisms. Two truncated human SP-D forms, wild-type (WT) and double mutant D325A+R343V, representing neck and carbohydrate recognition domains are compared in this study. Whereas both WT and D325A+R343V bind to isolated glycosylated HA, WT does not inhibit IAV in neutralization assays; in contrast, D325A+R343V neutralization compares well with that of full-length native SP-D. To elucidate the mechanism for these biochemical observations, we have determined crystal structures of D325A+R343V in the presence and absence of a viral nonamannoside (Man9). On the basis of the D325A+R343V-Man9 structure and other crystallographic data, models of complexes between HA and WT or D325A+R343V were produced and subjected to molecular dynamics. Simulations reveal that whereas WT and D325A+R343V both block the sialic acid receptor site of HA, the D325A+R343V complex is more stable, with stronger binding caused by additional hydrogen bonds and hydrophobic interactions with HA residues. Furthermore, the blocking mechanism of HA differs for WT and D325A+R343V because of alternate glycan binding modes. The combined results suggest a mechanism through which the mode of SP-D-HA interaction could significantly influence viral aggregation and neutralization. These studies provide the first atomic-level molecular view of an innate host defense lectin inhibiting its viral glycoprotein target.
Organizational Affiliation:
Beckman Institute and Department of Physics, University of Illinois at Urbana-Champaign , Urbana, Illinois 61801, United States.