The role of T56 in controlling the flexibility of the distal histidine in dehaloperoxidase-hemoglobin from Amphitrite ornata.
Jiang, S., Wright, I., Swartz, P., Franzen, S.(2013) Biochim Biophys Acta 1834: 2020-2029
- PubMed: 23792762
- DOI: https://doi.org/10.1016/j.bbapap.2013.06.005
- Primary Citation of Related Structures:
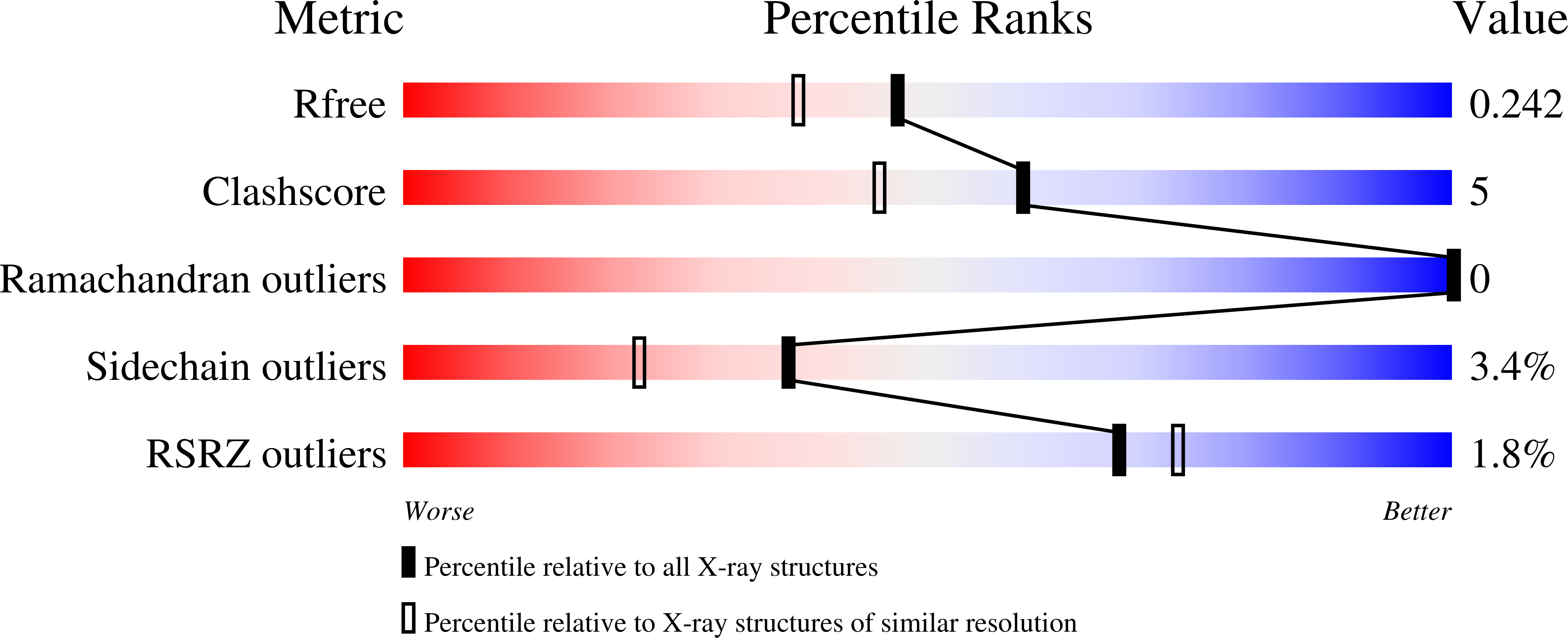
4JAC - PubMed Abstract:
The activation of dehaloperoxidase-hemoglobin (DHP) to form a ferryl intermediate requires the distal histidine, H55, to act as an acid base catalyst. The lack of ancillary amino acids in the distal pocket to assist in this process makes H55 even more important to the formation of active intermediates than in conventional peroxidases. Therefore, one can infer that the precise conformation H55 may greatly affect the enzymatic activity. Using site-direct mutagenesis at position T56, immediately adjacent to H55, we have confirmed that subtle changes in the conformation of H55 affect the catalytic efficiency of DHP. Mutating T56 to a smaller amino acid appears to permit H55 to rotate with relatively low barriers between conformations in the distal pocket, which may lead to an increase in catalytic activity. On the other hand, larger amino acids in the neighboring site appear to restrict the rotation of H55 due to the steric hindrance. In the case of T56V, which is an isosteric mutation, H55 appears less mobile, but forced to be closer to the heme iron than in wild type. Both proximity to the heme iron and flexibility of motion in some of the mutants can result in an increased catalytic rate, but can also lead to protein inactivation due to ligation of H55 to the heme iron, which is known as hemichrome formation. A balance of enzymatic rate and protein stability with respect to hemichrome formation appears to be optimum in wild type DHP (WT-DHP).
Organizational Affiliation:
Department of Chemistry, North Carolina State University, Raleigh, NC 27695, USA.