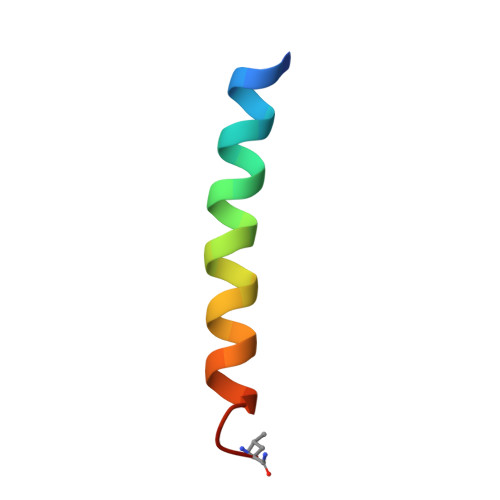
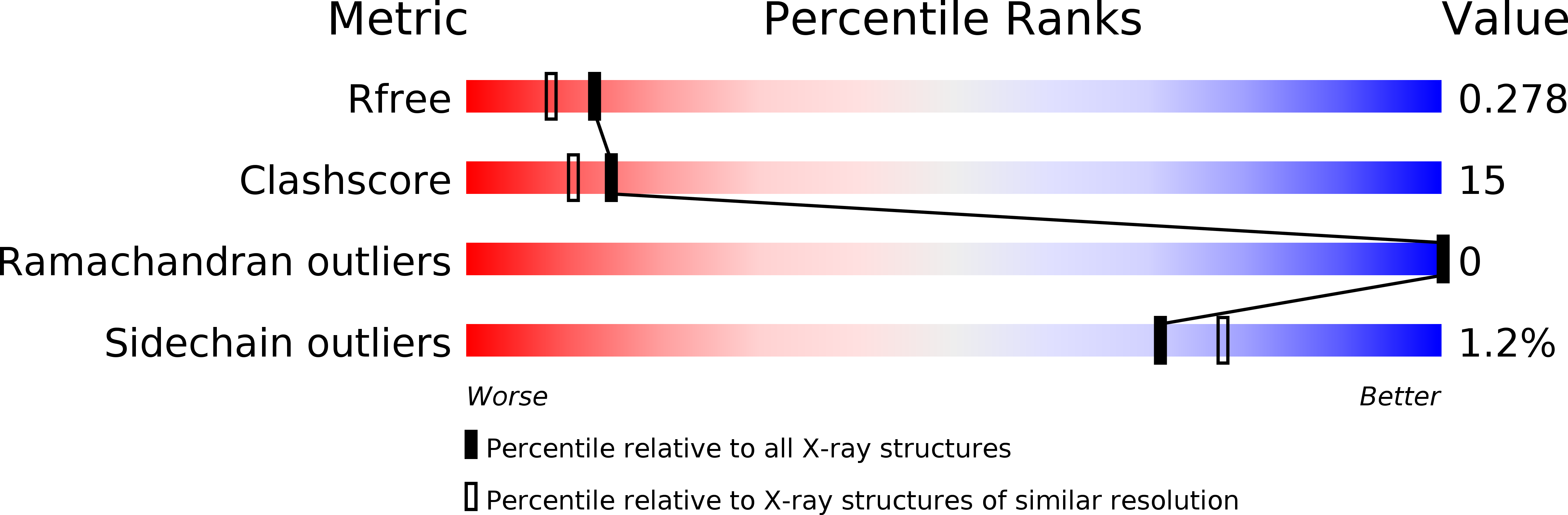Computational design of a protein crystal.
Lanci, C.J., Macdermaid, C.M., Kang, S.G., Acharya, R., North, B., Yang, X., Qiu, X.J., Degrado, W.F., Saven, J.G.(2012) Proc Natl Acad Sci U S A 109: 7304-7309
- PubMed: 22538812
- DOI: https://doi.org/10.1073/pnas.1112595109
- Primary Citation of Related Structures:
3V86, 4DAC - PubMed Abstract:
Protein crystals have catalytic and materials applications and are central to efforts in structural biology and therapeutic development. Designing predetermined crystal structures can be subtle given the complexity of proteins and the noncovalent interactions that govern crystallization. De novo protein design provides an approach to engineer highly complex nanoscale molecular structures, and often the positions of atoms can be programmed with sub-Å precision. Herein, a computational approach is presented for the design of proteins that self-assemble in three dimensions to yield macroscopic crystals. A three-helix coiled-coil protein is designed de novo to form a polar, layered, three-dimensional crystal having the P6 space group, which has a "honeycomb-like" structure and hexameric channels that span the crystal. The approach involves: (i) creating an ensemble of crystalline structures consistent with the targeted symmetry; (ii) characterizing this ensemble to identify "designable" structures from minima in the sequence-structure energy landscape and designing sequences for these structures; (iii) experimentally characterizing candidate proteins. A 2.1 Å resolution X-ray crystal structure of one such designed protein exhibits sub-Å agreement [backbone root mean square deviation (rmsd)] with the computational model of the crystal. This approach to crystal design has potential applications to the de novo design of nanostructured materials and to the modification of natural proteins to facilitate X-ray crystallographic analysis.
Organizational Affiliation:
Department of Chemistry, University of Pennsylvania, Philadelphia, PA 19104-6323, USA.