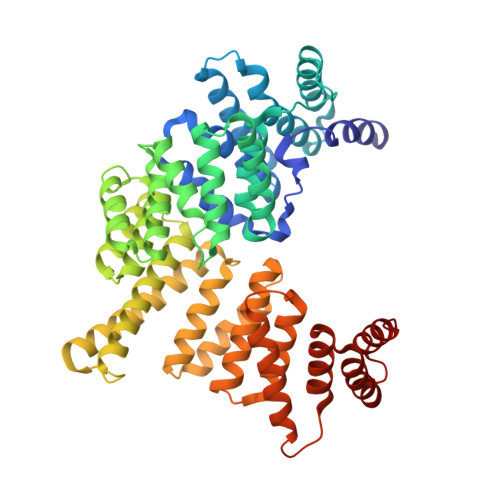
TRNA Binding, Structure, and Localization of the Human Interferon-Induced Protein Ifit5.
Katibah, G.E., Lee, H.J., Huizar, J.P., Vogan, J.M., Alber, T., Collins, K.(2013) Mol Cell 49: 743
- PubMed: 23317505
- DOI: https://doi.org/10.1016/j.molcel.2012.12.015
- Primary Citation of Related Structures:
3ZGQ - PubMed Abstract:
Interferon-induced proteins, including the largely uncharacterized interferon-induced tetratricopeptide repeat (IFIT) protein family, provide defenses against pathogens. Differing from expectations for tetratricopeptide repeat (TPR) proteins and from human IFIT1, IFIT2, and IFIT3, we show that human IFIT5 recognizes cellular RNA instead of protein partners. In vivo and in vitro, IFIT5 bound to endogenous 5'-phosphate-capped RNAs, including transfer RNAs. The crystal structure of IFIT5 revealed a convoluted intramolecular packing of eight TPRs as a fold that we name the TPR eddy. Additional, non-TPR structural elements contribute to an RNA binding cleft. Instead of general cytoplasmic distribution, IFIT5 concentrated in actin-rich protrusions from the apical cell surface colocalized with the RNA-binding retinoic acid-inducible gene-I (RIG-I). These findings establish compartmentalized cellular RNA binding activity as a mechanism for IFIT5 function and reveal the TPR eddy as a scaffold for RNA recognition.
Organizational Affiliation:
Department of Molecular and Cell Biology, University of California, Berkeley, Berkeley, CA 94720, USA.