Specialized peptidoglycan hydrolases sculpt the intra-bacterial niche of predatory Bdellovibrio and increase population fitness.
Lerner, T.R., Lovering, A.L., Bui, N.K., Uchida, K., Aizawa, S., Vollmer, W., Sockett, R.E.(2012) PLoS Pathog 8: e1002524-e1002524
- PubMed: 22346754
- DOI: https://doi.org/10.1371/journal.ppat.1002524
- Primary Citation of Related Structures:
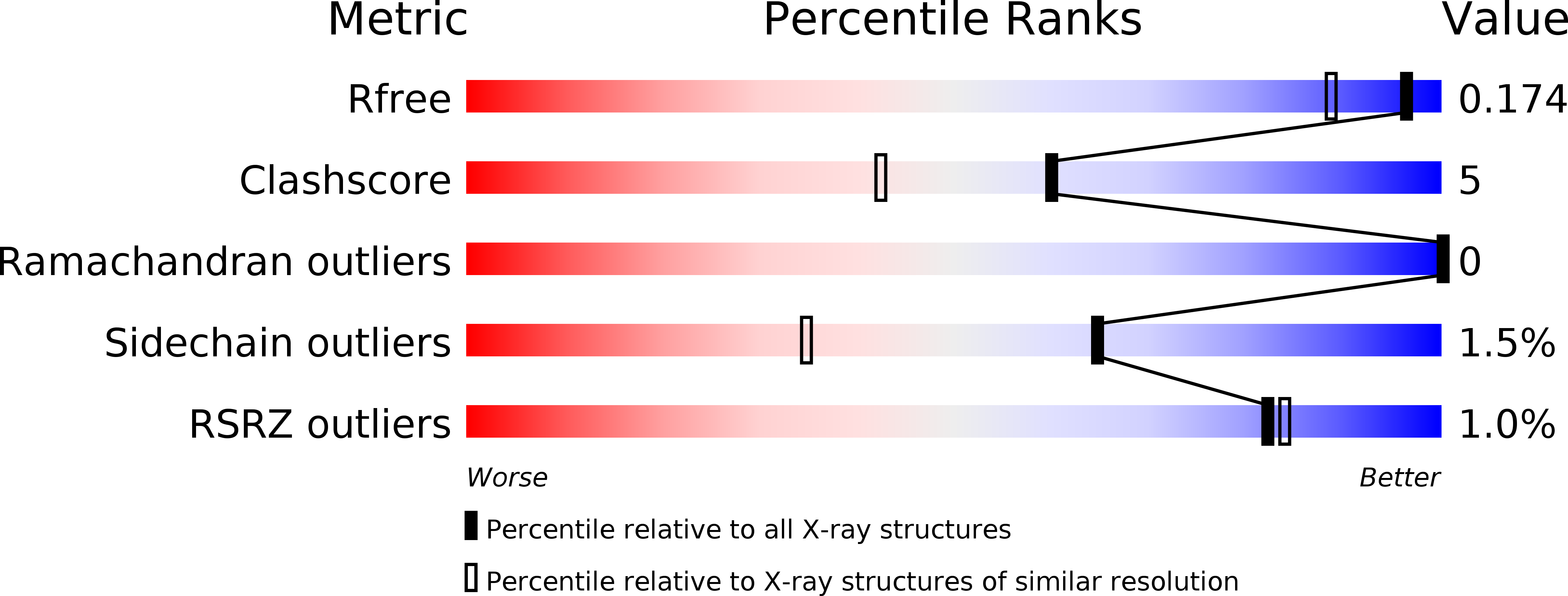
3V39 - PubMed Abstract:
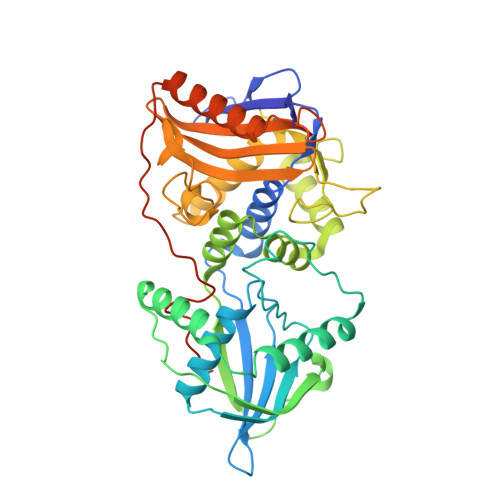
Bdellovibrio are predatory bacteria that have evolved to invade virtually all gram-negative bacteria, including many prominent pathogens. Upon invasion, prey bacteria become rounded up into an osmotically stable niche for the Bdellovibrio, preventing further superinfection and allowing Bdellovibrio to replicate inside without competition, killing the prey bacterium and degrading its contents. Historically, prey rounding was hypothesized to be associated with peptidoglycan (PG) metabolism; we found two Bdellovibrio genes, bd0816 and bd3459, expressed at prey entry and encoding proteins with limited homologies to conventional dacB/PBP4 DD-endo/carboxypeptidases (responsible for peptidoglycan maintenance during growth and division). We tested possible links between Bd0816/3459 activity and predation. Bd3459, but not an active site serine mutant protein, bound β-lactam, exhibited DD-endo/carboxypeptidase activity against purified peptidoglycan and, importantly, rounded up E. coli cells upon periplasmic expression. A ΔBd0816 ΔBd3459 double mutant invaded prey more slowly than the wild type (with negligible prey cell rounding) and double invasions of single prey by more than one Bdellovibrio became more frequent. We solved the crystal structure of Bd3459 to 1.45 Å and this revealed predation-associated domain differences to conventional PBP4 housekeeping enzymes (loss of the regulatory domain III, alteration of domain II and a more exposed active site). The Bd3459 active site (and by similarity the Bd0816 active site) can thus accommodate and remodel the various bacterial PGs that Bdellovibrio may encounter across its diverse prey range, compared to the more closed active site that "regular" PBP4s have for self cell wall maintenance. Therefore, during evolution, Bdellovibrio peptidoglycan endopeptidases have adapted into secreted predation-specific proteins, preventing wasteful double invasion, and allowing activity upon the diverse prey peptidoglycan structures to sculpt the prey cell into a stable intracellular niche for replication.
Organizational Affiliation:
Centre for Genetics and Genomics, School of Biology, University of Nottingham, Medical School, Nottingham, United Kingdom.