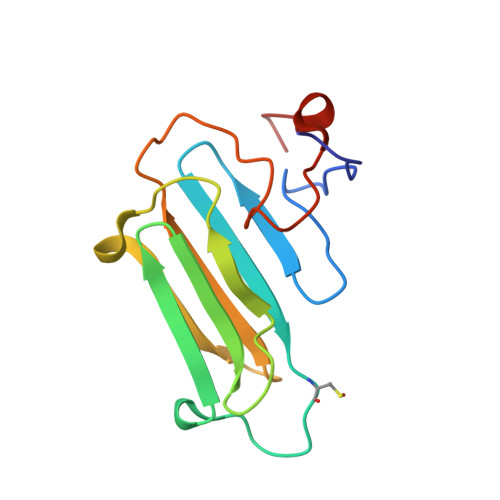
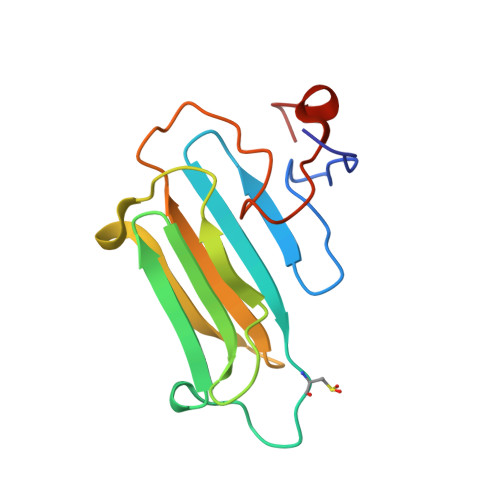
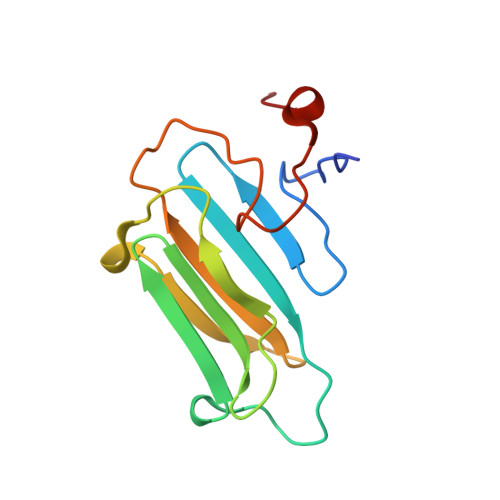
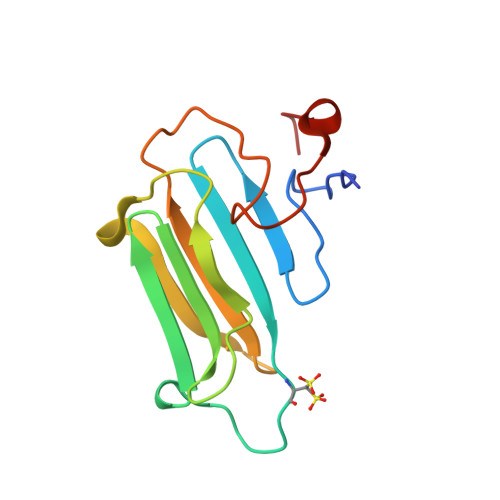
Structural Features and Chaperone Activity of the NudC Protein Family.
Zheng, M., Cierpicki, T., Burdette, A.J., Utepbergenov, D., Janczyk, P.L., Derewenda, U., Stukenberg, P.T., Caldwell, K.A., Derewenda, Z.S.(2011) J Mol Biol 409: 722-741
- PubMed: 21530541
- DOI: https://doi.org/10.1016/j.jmb.2011.04.018
- Primary Citation of Related Structures:
3QOR - PubMed Abstract:
The NudC family consists of four conserved proteins with representatives in all eukaryotes. The archetypal nudC gene from Aspergillus nidulans is a member of the nud gene family that is involved in the maintenance of nuclear migration. This family also includes nudF, whose human orthologue, Lis1, codes for a protein essential for brain cortex development. Three paralogues of NudC are known in vertebrates: NudC, NudC-like (NudCL), and NudC-like 2 (NudCL2). The fourth distantly related member of the family, CML66, contains a NudC-like domain. The three principal NudC proteins have no catalytic activity but appear to play as yet poorly defined roles in proliferating and dividing cells. We present crystallographic and NMR studies of the human NudC protein and discuss the results in the context of structures recently deposited by structural genomics centers (i.e., NudCL and mouse NudCL2). All proteins share the same core CS domain characteristic of proteins acting either as cochaperones of Hsp90 or as independent small heat shock proteins. However, while NudC and NudCL dimerize via an N-terminally located coiled coil, the smaller NudCL2 lacks this motif and instead dimerizes as a result of unique domain swapping. We show that NudC and NudCL, but not NudCL2, inhibit the aggregation of several target proteins, consistent with an Hsp90-independent heat shock protein function. Importantly, and in contrast to several previous reports, none of the three proteins is able to form binary complexes with Lis1. The availability of structural information will be of help in further studies on the cellular functions of the NudC family.
Organizational Affiliation:
Department of Molecular Physiology and Biological Physics, University of Virginia School of Medicine, Charlottesville, VA 22908, USA.