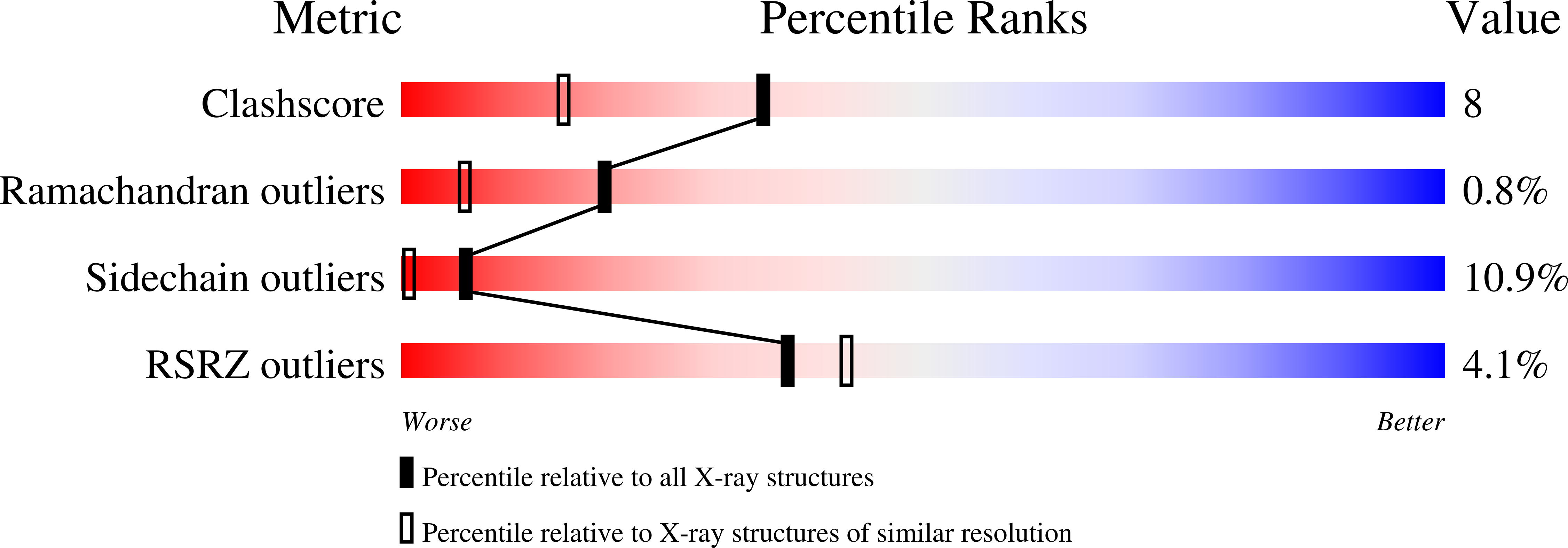
Site-directed mutants of pseudoazurin: explanation of increased redox potentials from X-ray structures and from calculation of redox potential differences.
Libeu, C.A., Kukimoto, M., Nishiyama, M., Horinouchi, S., Adman, E.T.(1997) Biochemistry 36: 13160-13179
- PubMed: 9341204
- DOI: https://doi.org/10.1021/bi9704111
- Primary Citation of Related Structures:
3PAZ, 4PAZ, 5PAZ, 6PAZ, 7PAZ, 8PAZ - PubMed Abstract:
In order to understand the origins of differences in redox potentials among cupredoxins (small blue type I copper-containing proteins that reversibly change oxidation state and interact with redox partners), we have determined the structures of the native and two mutants (P80A and P80I) of pseudoazurin from Alcaligenes faecalis S-6 in oxidized and reduced forms at resolutions of 2.2 A in the worst case and 1.6 A in the best case. The P80A mutation creates a surface pocket filled by a new water molecule, whereas the P80I mutant excludes this water. Distinct patterns of change occur in response to reduction for all three molecules: the copper position shifts, Met 7 and Pro 35 move, and the relative orientations of residues 81 to 16, 18 to the amide planes of 77 and 86, all change. Systematic changes in the weak electrostatic interactions seen in the structures of different oxidation states can explain the Met 7/Pro 35 structural differences as well as some fluctuating solvent positions. Overall displacement parameters increase reversibly upon reduction. The reduced forms are slightly expanded over the oxidized forms. The geometries of the mutants become more trigonal in their reduced forms, consistent with higher redox potentials (+409 mV for P80A and +450 mV for P80I). Calculations of the differences in redox potentials, using POLARIS, reveal that a water unique to the P80A mutant is required (with correctly oriented hydrogens) to approximate the observed difference in redox potential. The POLARIS calculations suggest that the reduced forms are additionally stabilized through changes in the solvation of the copper center, specifically via the amides of residues 16, 39, 41, 79, and 80 which interact with either Phe 18, Met 86, or Cys 78. The redox potential of P80A is increased largely due to solvation effects, whereas the redox potential of P80I is increased largely due to geometrical effects.
Organizational Affiliation:
Department of Biological Structure, University of Washington, Box 357420, Seattle, Washington 98195-7420, USA.