Structural Studies of the Tandem Tudor Domains of Fragile X Mental Retardation Related Proteins FXR1 and FXR2.
Adams-Cioaba, M.A., Guo, Y., Bian, C., Amaya, M.F., Lam, R., Wasney, G.A., Vedadi, M., Xu, C., Min, J.(2010) PLoS One 5: e13559-e13559
- PubMed: 21072162
- DOI: https://doi.org/10.1371/journal.pone.0013559
- Primary Citation of Related Structures:
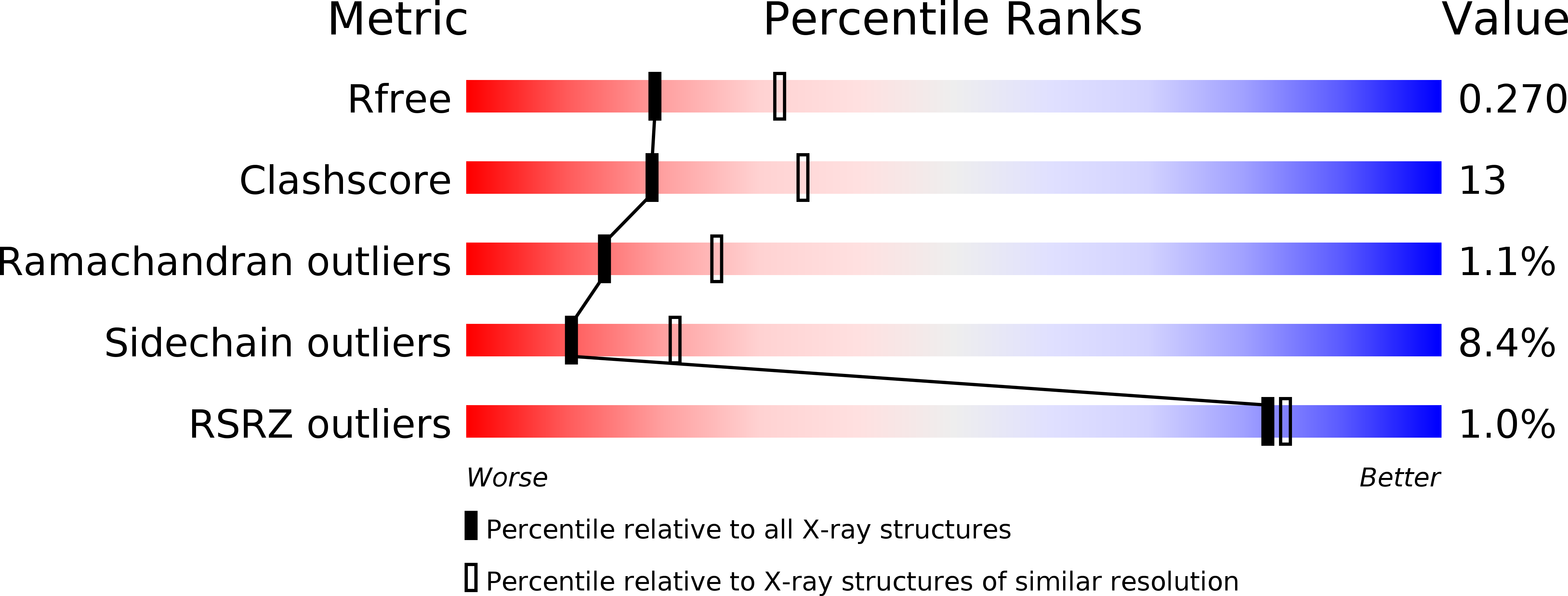
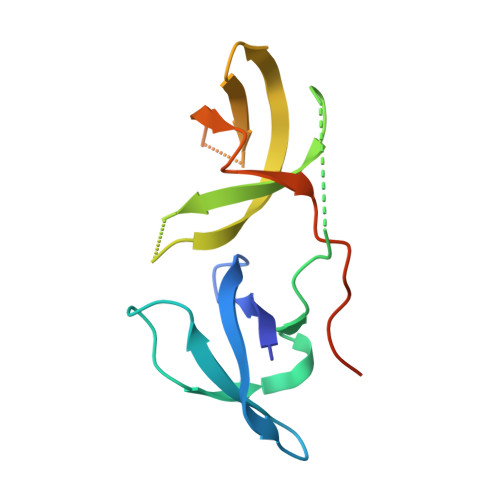
3H8Z, 3O8V - PubMed Abstract:
Expansion of the CGG trinucleotide repeat in the 5'-untranslated region of the FMR1, fragile X mental retardation 1, gene results in suppression of protein expression for this gene and is the underlying cause of Fragile X syndrome. In unaffected individuals, the FMRP protein, together with two additional paralogues (Fragile X Mental Retardation Syndrome-related Protein 1 and 2), associates with mRNA to form a ribonucleoprotein complex in the nucleus that is transported to dendrites and spines of neuronal cells. It is thought that the fragile X family of proteins contributes to the regulation of protein synthesis at sites where mRNAs are locally translated in response to stimuli.
Organizational Affiliation:
Structural Genomics Consortium, University of Toronto, Toronto, Ontario, Canada.