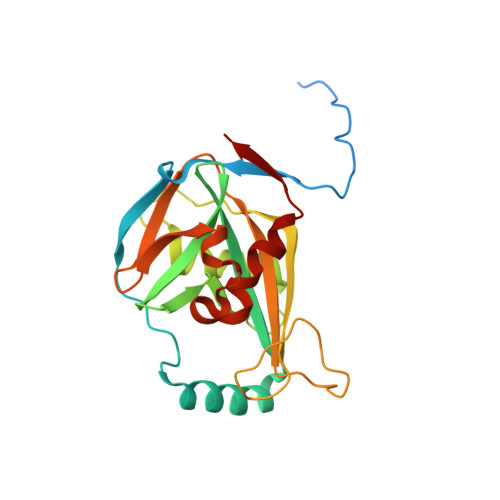
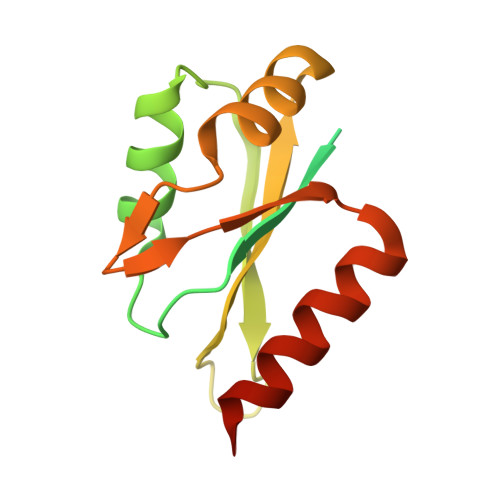
Crystal Structure of the Complex between the 25 kDa Subunit and the 59 kDa Subunit (RRM domain) of Human Cleavage Factor Im
Tresaugues, L., Welin, M., Arrowsmith, C.H., Berglund, H., Bountra, C., Collins, R., Edwards, A.M., Flodin, S., Flores, A., Graslund, S., Hammarstrom, M., Johansson, I., Karlberg, T., Kol, S., Kotenyova, T., Moche, M., Nielsen, T.K., Nyman, T., Persson, C., Schuler, H., Schutz, P., Siponen, M.I., Thorsell, A.G., van der Berg, S., Wahlberg, E., Weigelt, J., Wisniewska, M., Nordlund, P.To be published.