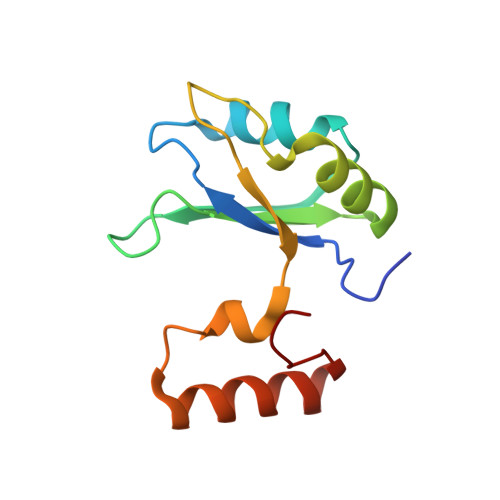
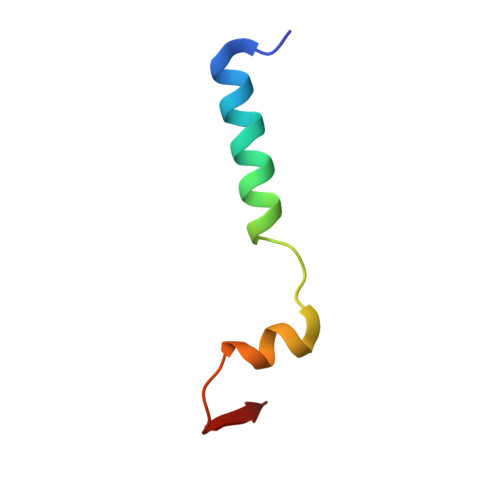
Branch Recognition by SF3b14
Schellenberg, M.J., Dul, E.L., MacMillan, A.M.(2011) RNA 17: 155-165
- PubMed: 21062891
- DOI: https://doi.org/10.1261/rna.2224411
- Primary Citation of Related Structures:
3LQV - PubMed Abstract:
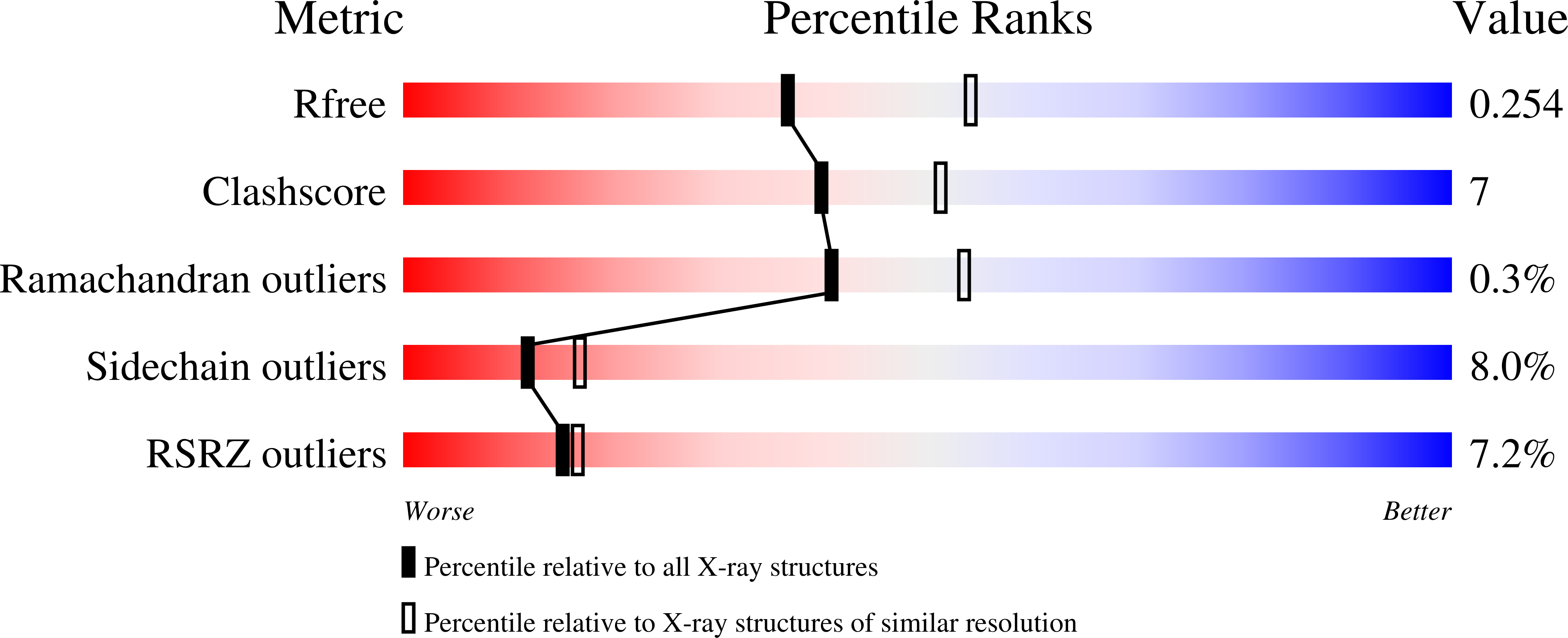
Human p14 (SF3b14), a component of the spliceosomal U2 snRNP, interacts directly with the pre-mRNA branch adenosine within the context of the bulged duplex formed between the pre-mRNA branch region and U2 snRNA. This association occurs early in spliceosome assembly and persists within the fully assembled spliceosome. Analysis of the crystal structure of a complex containing p14 and a peptide derived from p14-associated SF3b155 combined with the results of cross-linking studies has suggested that the branch nucleotide interacts with a pocket on a non-canonical RNA binding surface formed by the complex. Here we report a structural model of the p14 · bulged duplex interaction based on a combination of X-ray crystallography of an adenine p14/SF3b155 peptide complex, biochemical comparison of a panel of disulfide cross-linked protein-RNA complexes, and small-angle X-ray scattering (SAXS). These studies reveal specific recognition of the branch adenosine within the p14 pocket and establish the orientation of the bulged duplex RNA bound on the protein surface. The intimate association of one surface of the bulged duplex with the p14/SF3b155 peptide complex described by this model buries the branch nucleotide at the interface and suggests that p14 · duplex interaction must be disrupted before the first step of splicing.
Organizational Affiliation:
Department of Biochemistry, School of Molecular and Systems Medicine, University of Alberta, Edmonton, Alberta, Canada.