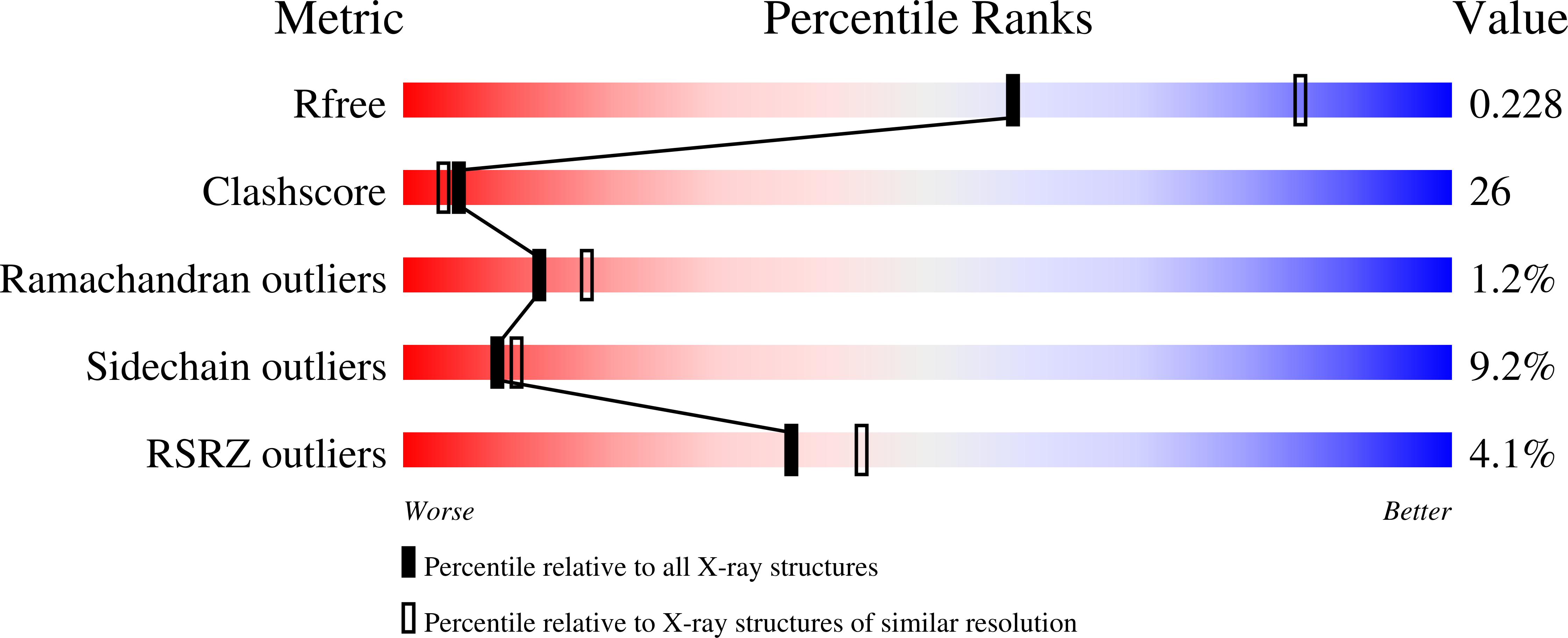
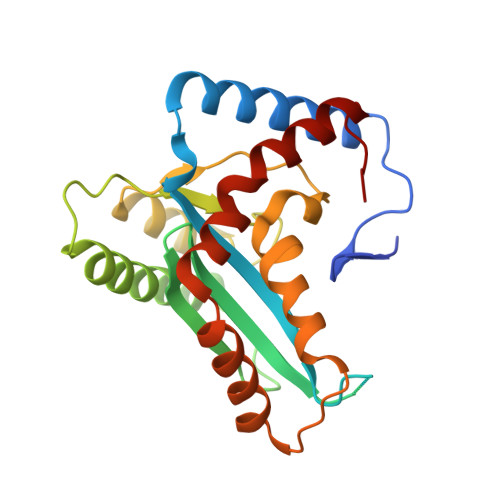
Crystal structure of the human Hsmar1-derived transposase domain in the DNA repair enzyme Metnase.
Goodwin, K.D., He, H., Imasaki, T., Lee, S.H., Georgiadis, M.M.(2010) Biochemistry 49: 5705-5713
- PubMed: 20521842
- DOI: https://doi.org/10.1021/bi100171x
- Primary Citation of Related Structures:
3K9J, 3K9K - PubMed Abstract:
Although the human genome is littered with sequences derived from the Hsmar1 transposon, the only intact Hsmar1 transposase gene exists within a chimeric SET-transposase fusion protein referred to as Metnase or SETMAR. Metnase retains many of the transposase activities including terminal inverted repeat (TIR) specific DNA-binding activity, DNA cleavage activity, albeit uncoupled from TIR-specific binding, and the ability to form a synaptic complex. However, Metnase has evolved as a DNA repair protein that is specifically involved in nonhomologous end joining. Here, we present two crystal structures of the transposase catalytic domain of Metnase revealing a dimeric enzyme with unusual active site plasticity that may be involved in modulating metal binding. We show through characterization of a dimerization mutant, F460K, that the dimeric form of the enzyme is required for its DNA cleavage, DNA-binding, and nonhomologous end joining activities. Of significance is the conservation of F460 along with residues that we propose may be involved in the modulation of metal binding in both the predicted ancestral Hsmar1 transposase sequence as well as in the modern enzyme. The Metnase transposase has been remarkably conserved through evolution; however, there is a clustering of substitutions located in alpha helices 4 and 5 within the putative DNA-binding site, consistent with loss of transposition specific DNA cleavage activity and acquisition of DNA repair specific cleavage activity.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, Indiana University School of Medicine, Indianapolis, Indiana 46202, USA.