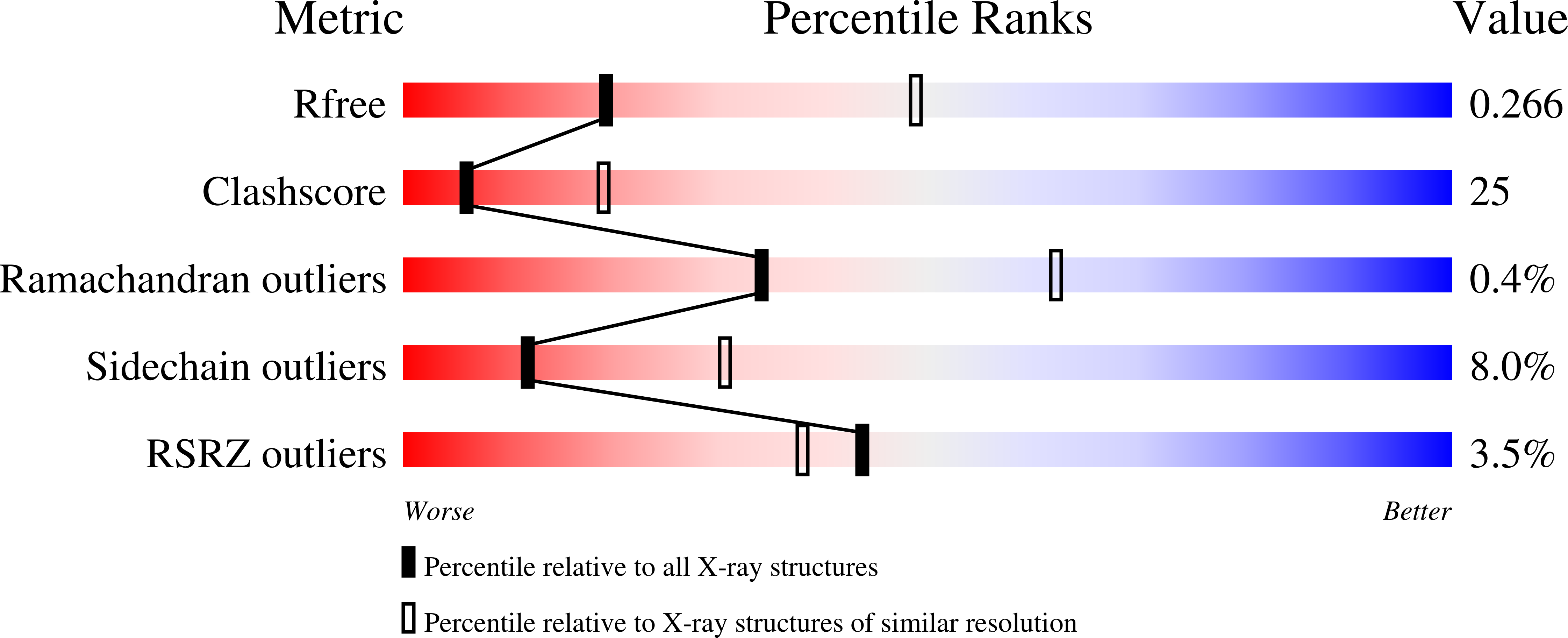
Mitochondrial localization and structure-based phosphate activation mechanism of Glutaminase C with implications for cancer metabolism.
Cassago, A., Ferreira, A.P., Ferreira, I.M., Fornezari, C., Gomes, E.R., Greene, K.S., Pereira, H.M., Garratt, R.C., Dias, S.M., Ambrosio, A.L.(2012) Proc Natl Acad Sci U S A 109: 1092-1097
- PubMed: 22228304
- DOI: https://doi.org/10.1073/pnas.1112495109
- Primary Citation of Related Structures:
3SS3, 3SS4, 3SS5 - PubMed Abstract:
Glutamine is an essential nutrient for cancer cell proliferation, especially in the context of citric acid cycle anaplerosis. In this manuscript we present results that collectively demonstrate that, of the three major mammalian glutaminases identified to date, the lesser studied splice variant of the gene gls, known as Glutaminase C (GAC), is important for tumor metabolism. We show that, although levels of both the kidney-type isoforms are elevated in tumor vs. normal tissues, GAC is distinctly mitochondrial. GAC is also most responsive to the activator inorganic phosphate, the content of which is supposedly higher in mitochondria subject to hypoxia. Analysis of X-ray crystal structures of GAC in different bound states suggests a mechanism that introduces the tetramerization-induced lifting of a "gating loop" as essential for the phosphate-dependent activation process. Surprisingly, phosphate binds inside the catalytic pocket rather than at the oligomerization interface. Phosphate also mediates substrate entry by competing with glutamate. A greater tendency to oligomerize differentiates GAC from its alternatively spliced isoform and the cycling of phosphate in and out of the active site distinguishes it from the liver-type isozyme, which is known to be less dependent on this ion.
Organizational Affiliation:
Laboratório Nacional de Biociências, Centro Nacional de Pesquisa em Energia e Materiais, Campinas-SP 13083-970, Campinas, Brazil.