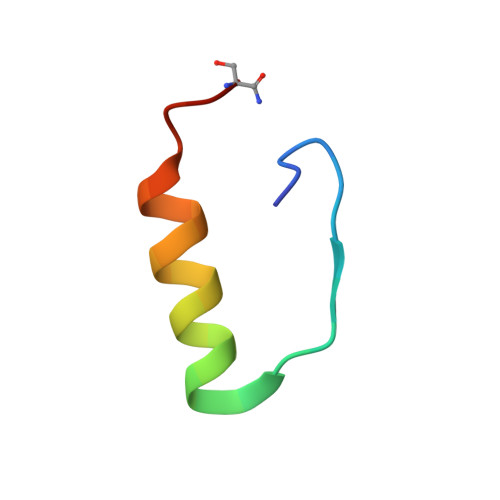
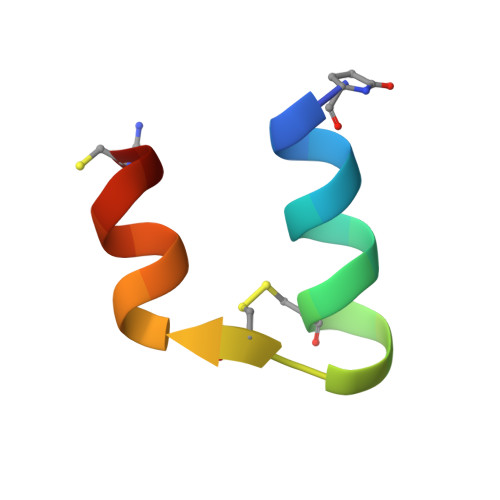
Solution structure, aggregation behavior, and flexibility of human relaxin-2.
Haugaard-Kedstrom, L.M., Hossain, M.A., Daly, N.L., Bathgate, R.A., Rinderknecht, E., Wade, J.D., Craik, D.J., Rosengren, K.J.(2015) ACS Chem Biol 10: 891-900
- PubMed: 25547165
- DOI: https://doi.org/10.1021/cb500918v
- Primary Citation of Related Structures:
2MV1 - PubMed Abstract:
Relaxin is a member of the relaxin/insulin peptide hormone superfamily and is characterized by a two-chain structure constrained by three disulfide bonds. Relaxin is a pleiotropic hormone and involved in a number of physiological and pathogenic processes, including collagen and cardiovascular regulation and tissue remodelling during pregnancy and cancer. Crystallographic and ultracentrifugation experiments have revealed that the human form of relaxin, H2 relaxin, self-associates into dimers, but the significance of this is poorly understood. Here, we present the NMR structure of a monomeric, amidated form of H2 relaxin and compare its features and behavior in solution to those of native H2 relaxin. The overall structure of H2 relaxin is retained in the monomeric form. H2 relaxin amide is fully active at the relaxin receptor RXFP1 and thus dimerization is not required for biological activity. Analysis of NMR chemical shifts and relaxation parameters identified internal motion in H2 relaxin at the pico-nanosecond and milli-microsecond time scales, which is commonly seen in other relaxin and insulin peptides and might be related to function.
Organizational Affiliation:
□School of Natural Sciences, Linnaeus University, SE-391 82 Kalmar, Sweden.