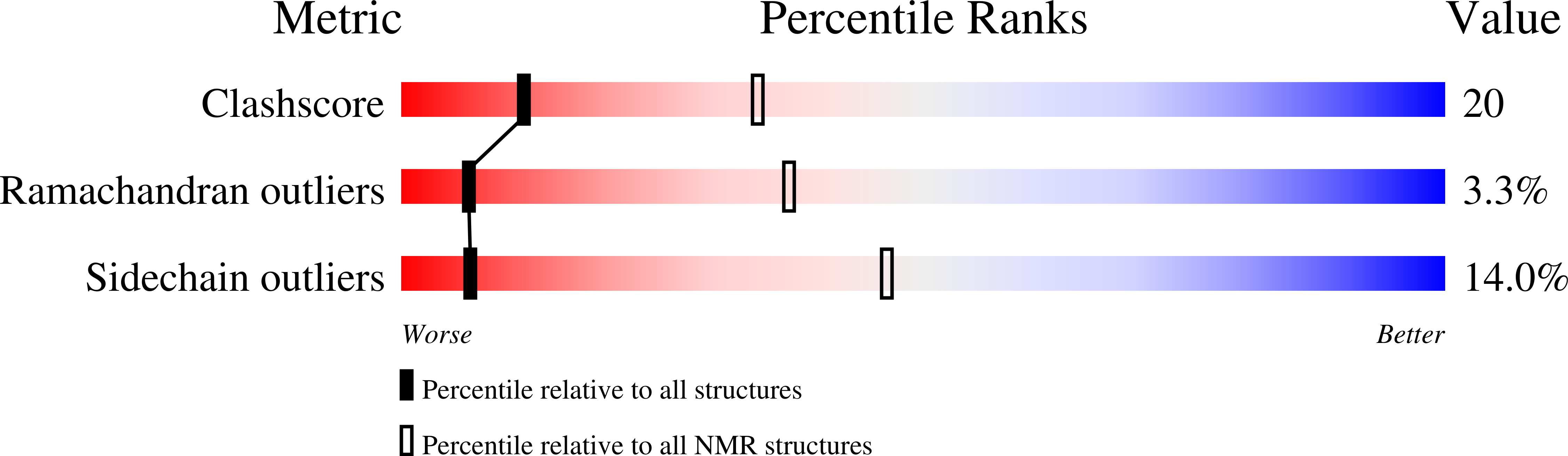Structural studies of FF domains of the transcription factor CA150 provide insights into the organization of FF domain tandem arrays.
Murphy, J.M., Hansen, D.F., Wiesner, S., Muhandiram, D.R., Borg, M., Smith, M.J., Sicheri, F., Kay, L.E., Forman-Kay, J.D., Pawson, T.(2009) J Mol Biol 393: 409-424
- PubMed: 19715701
- DOI: https://doi.org/10.1016/j.jmb.2009.08.049
- Primary Citation of Related Structures:
2KIS - PubMed Abstract:
FF domains are poorly understood protein interaction modules that are present within eukaryotic transcription factors, such as CA150 (TCERG-1). The CA150 FF domains have been shown to mediate interactions with the phosphorylated C-terminal domain of RNA polymerase II (phosphoCTD) and a multitude of transcription factors and RNA processing proteins, and may therefore have a central role in organizing transcription. FF domains occur in tandem arrays of up to six domains, although it is not known whether they adopt higher-order structures. We have used the CA150 FF1+FF2 domains as a model system to examine whether tandem FF domains form higher-order structures in solution using NMR spectroscopy. In the solution structure of FF1 fused to the linker that joins FF1 to FF2, we observed that the highly conserved linker peptide is ordered and forms a helical extension of helix alpha3, suggesting that the interdomain linker might have a role in orientating FF1 relative to FF2. However, examination of the FF1+FF2 domains using relaxation NMR experiments revealed that although these domains are not rigidly orientated relative to one another, they do not tumble independently. Thus, the FF1+FF2 structure conforms to a dumbbell-shape in solution, where the helical interdomain linker maintains distance between the two dynamic FF domains without cementing their relative orientations. This model for FF domain organization within tandem arrays suggests a general mechanism by which individual FF domains can manoeuvre to achieve optimal recognition of flexible binding partners, such as the intrinsically-disordered phosphoCTD.
Organizational Affiliation:
Samuel Lunenfeld Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada. jamesm@wehi.edu.au