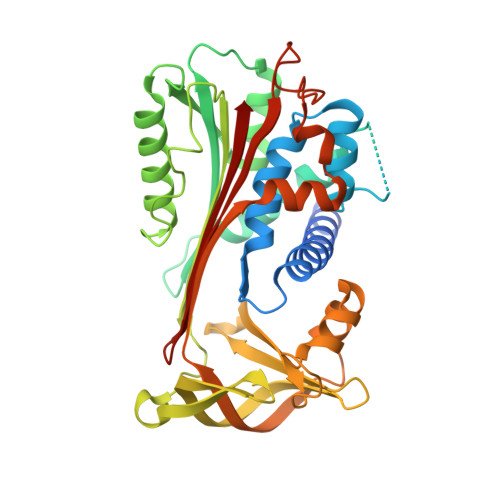
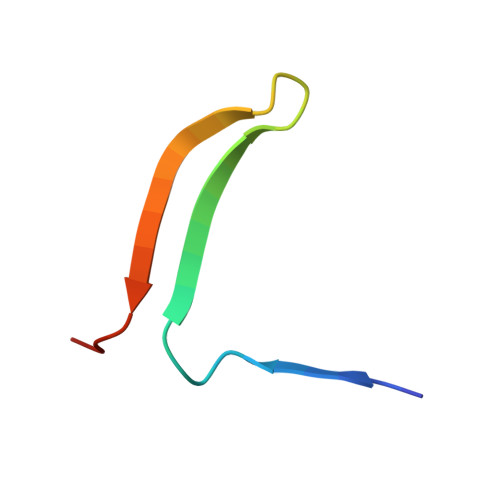
X-ray crystal structure of MENT: evidence for functional loop-sheet polymers in chromatin condensation.
McGowan, S., Buckle, A.M., Irving, J.A., Ong, P.C., Bashtannyk-Puhalovich, T.A., Kan, W.T., Henderson, K.N., Bulynko, Y.A., Popova, E.Y., Smith, A.I., Bottomley, S.P., Rossjohn, J., Grigoryev, S.A., Pike, R.N., Whisstock, J.C.(2006) EMBO J 25: 3144-3155
- PubMed: 16810322
- DOI: https://doi.org/10.1038/sj.emboj.7601201
- Primary Citation of Related Structures:
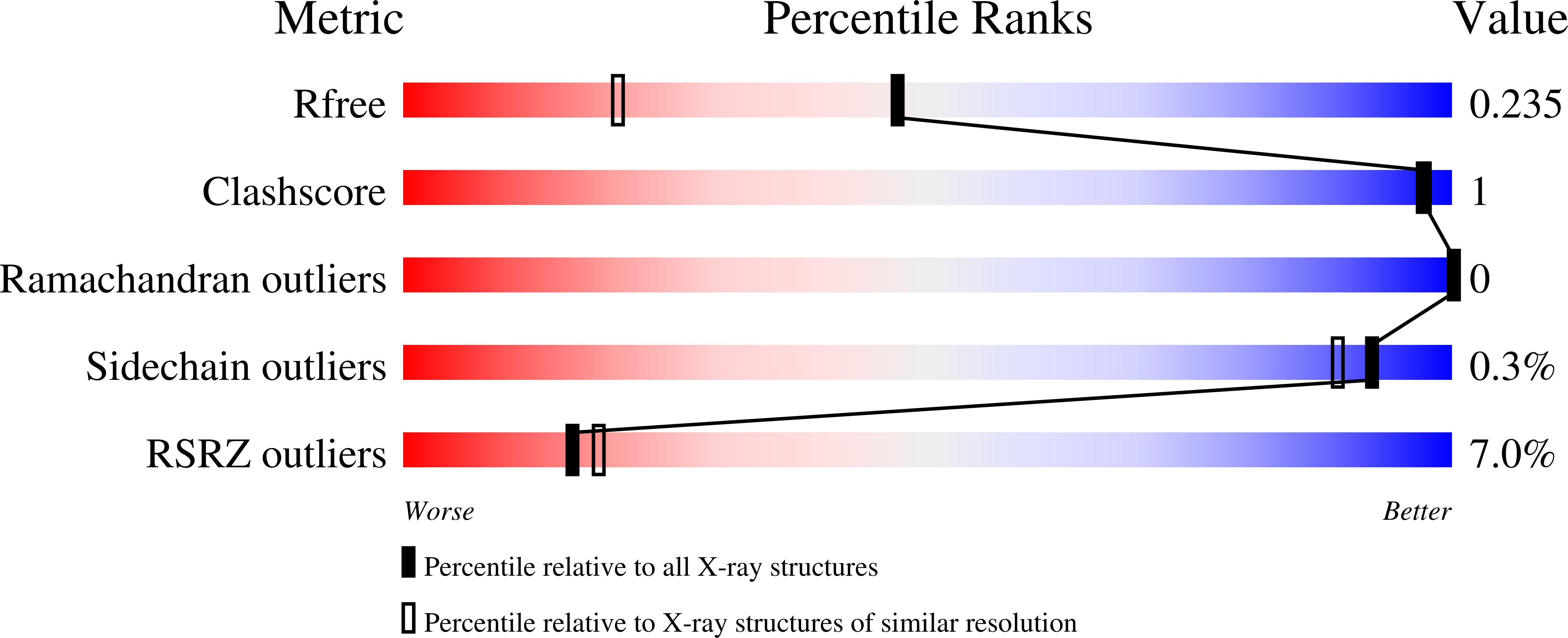
2DUT, 2H4P, 2H4Q, 2H4R - PubMed Abstract:
Most serpins are associated with protease inhibition, and their ability to form loop-sheet polymers is linked to conformational disease and the human serpinopathies. Here we describe the structural and functional dissection of how a unique serpin, the non-histone architectural protein, MENT (Myeloid and Erythroid Nuclear Termination stage-specific protein), participates in DNA and chromatin condensation. Our data suggest that MENT contains at least two distinct DNA-binding sites, consistent with its simultaneous binding to the two closely juxtaposed linker DNA segments on a nucleosome. Remarkably, our studies suggest that the reactive centre loop, a region of the MENT molecule essential for chromatin bridging in vivo and in vitro, is able to mediate formation of a loop-sheet oligomer. These data provide mechanistic insight into chromatin compaction by a non-histone architectural protein and suggest how the structural plasticity of serpins has adapted to mediate physiological, rather than pathogenic, loop-sheet linkages.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, Monash University, Clayton, Victoria, Australia.