Structure of PITPbeta in Complex with Phosphatidylcholine: Comparison of Structure and Lipid Transfer to Other PITP Isoforms.
Vordtriede, P.B., Doan, C.N., Tremblay, J.M., Helmkamp, G.M., Yoder, M.D.(2005) Biochemistry 44: 14760-14771
- PubMed: 16274224
- DOI: https://doi.org/10.1021/bi051191r
- Primary Citation of Related Structures:
2A1L - PubMed Abstract:
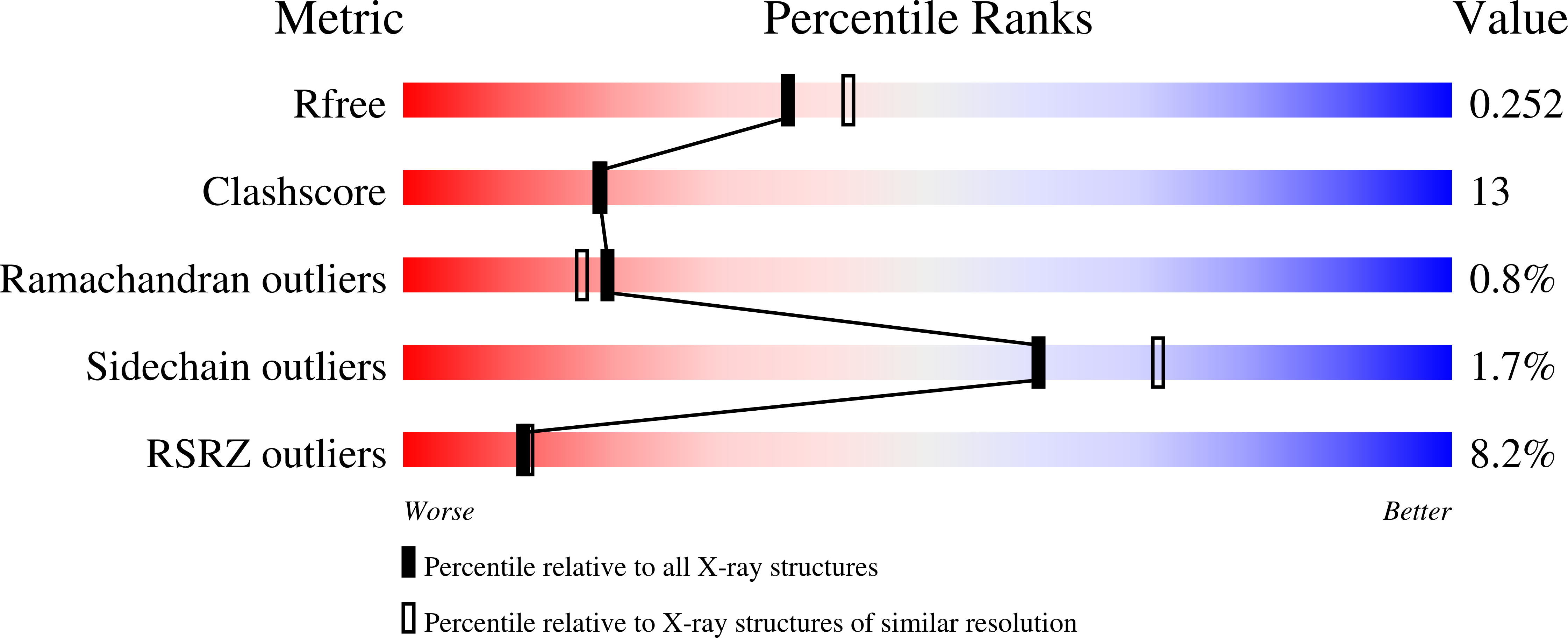
Phosphatidylinositol transfer protein (PITP) is a ubiquitous eukaryotic protein that preferentially binds either phosphatidylinositol or phosphatidylcholine and catalyzes the exchange of these lipids between membranes. Mammalian cytosolic PITPs include the ubiquitously expressed PITPalpha and PITPbeta isoforms (269-270 residues). The crystal structure of rat PITPbeta complexed to dioleoylphosphatidylcholine was determined to 2.18 A resolution with molecular replacement using rat PITPalpha (77% sequence identify) as the phasing model. A structure comparison of the alpha and beta isoforms reveals minimal differences in protein conformation, differences in acyl conformation in the two isoforms, and remarkable conservation of solvent structure around the bound lipid. A comparison of transfer activity by human and rat PITPs, using small unilamellar vesicles with carefully controlled phospholipid composition, indicates that the beta isoforms have minimal differences in transfer preference between PtdIns and PtdCho when donor vesicles contain predominantly PtdCho. When PtdCho and PtdIns are present in equivalent concentrations in donor vesicles, PtdIns transfer occurs at approximately 3-fold the rate of PtdCho. The rat PITPbeta isoform clearly has the most diminished transfer rate of the four proteins studied. With the two rat isoforms, site-directed mutations of two locations within the lipid binding cavity that possess differing biochemical properties were characterized: I84alpha/F83beta and F225alpha/L224beta. The 225/224 locus is more critical in determining substrate specificity. Following the mutation of this locus to the other amino acid, the PtdCho transfer specific activity became PITPalpha (F225L) approximately PITPbeta and PITPbeta (L224F) approximately PITPalpha. The 225alpha/224beta locus plays a modest role in the specificity of both isoforms toward CerPCho.
Organizational Affiliation:
Division of Cell Biology and Biophysics, University of Missouri-Kansas City, Kansas City, Missouri 64110-2499, USA.