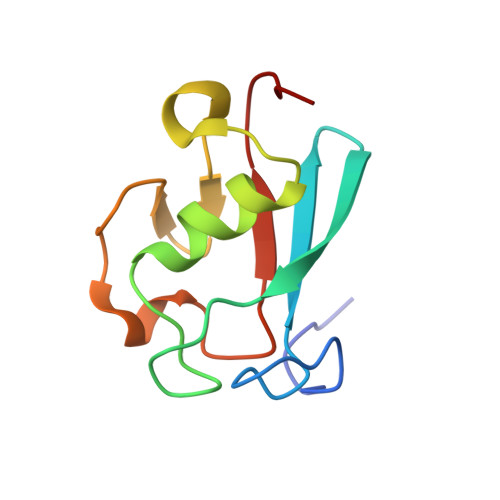
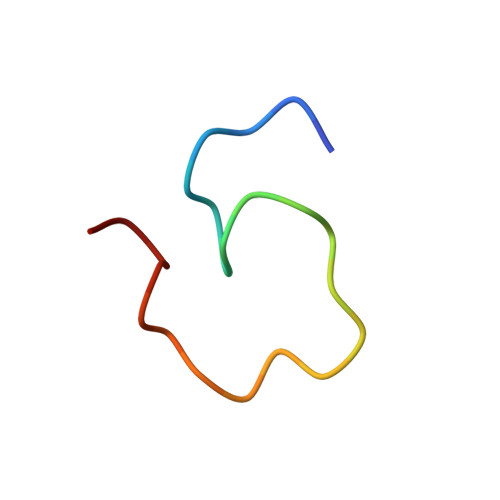
Nuclear Magnetic Resonance Structure of the Cytoplasmic Tail of Heparin Binding EGF-like Growth Factor (proHB-EGF-CT) Complexed with the Ubiquitin Homology Domain of Bcl-2-Associated Athanogene 1 from Mus musculus (mBAG-1-UBH).
Hung, K.W., Huang, H.W., Cho, C.C., Chang, S.C., Yu, C.(2014) Biochemistry 53: 1935-1946
- PubMed: 24628338
- DOI: https://doi.org/10.1021/bi5003019
- Primary Citation of Related Structures:
2M8S - PubMed Abstract:
The membrane form of heparin binding EGF-like growth factor (proHB-EGF) yields secreted HB-EGF and a membrane-anchored cytoplasmic tail (proHB-EGF-CT), which may be targeted to the nuclear membrane after a shedding stimulus. Bcl-2-associated athanogene 1 (BAG-1) accumulates in the nuclei and inhibits apoptosis in adenoma-derived cell lines. The maintenance of high levels of nuclear BAG-1 enhances cell survival. However, the ubiquitin homology domain of BAG-1 from Mus musculus (mBAG-1-UBH) is proposed to interact with proHB-EGF-CT, and this interaction may enhance the cytoprotection against the apoptosis inducer. The mechanism of the synergistic anti-apoptosis function of proHB-EGF-CT and mBAG-1-UBH is still unknown. We offer a hypothesis that proHB-EGF-CT can maintain high levels of nuclear BAG-1. In this study, we first report the three-dimensional nuclear magnetic resonance structure of proHB-EGF-CT complexed with mBAG-1-UBH. In the structure of the complex, the residues in the C-terminus and one turn between β-strands β1 and β2 of mBAG-1-UBH bind to two terminals of proHB-EGF-CT, which folds into a loop with end-to-end contact. This end-to-end folding of proHB-EGF-CT causes the basic amino acids to colocalize and form a positively charged groove. The dominant forces in the binding interface between proHB-EGF-CT and mBAG-1-UBH are charge-charge interactions. On the basis of our mutagenesis results, the basic amino acid cluster in the N-terminus of proHB-EGF-CT is the crucial binding site for mBAG-1-UBH, whereas another basic amino acid in the C-terminus facilitates this interaction. Interestingly, the mBAG-1-UBH binding region on the proHB-EGF-CT peptide is also involved in the region found to be important for nuclear envelope targeting, supporting the hypothesis that proHB-EGF-CT is most likely able to trigger the nuclear translocation of BAG-1 in keeping its level high.
Organizational Affiliation:
Instrumentation Center, National Tsing Hua University , Hsinchu 30013, Taiwan.