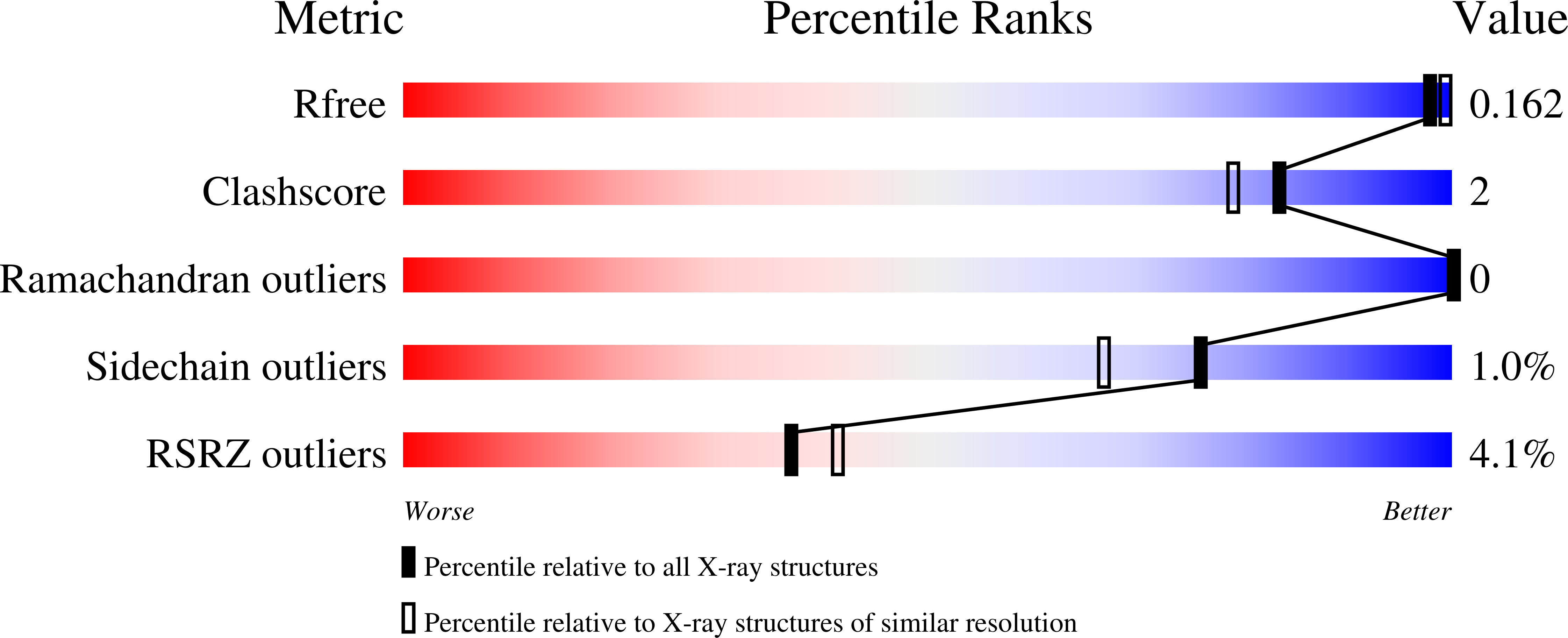
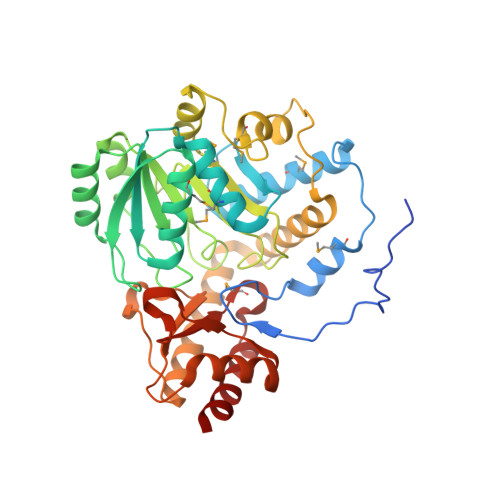
Crystal structure of an alanine-glyoxylate aminotransferase from Anabaena sp. at 1.70 A resolution reveals a noncovalently linked PLP cofactor
Han, G.W., Schwarzenbacher, R., Page, R., Jaroszewski, L., Abdubek, P., Ambing, E., Biorac, T., Canaves, J.M., Chiu, H.J., Dai, X., Deacon, A.M., DiDonato, M., Elsliger, M.A., Godzik, A., Grittini, C., Grzechnik, S.K., Hale, J., Hampton, E., Haugen, J., Hornsby, M., Klock, H.E., Koesema, E., Kreusch, A., Kuhn, P., Lesley, S.A., Levin, I., McMullan, D., McPhillips, T.M., Miller, M.D., Morse, A., Moy, K., Nigoghossian, E., Ouyang, J., Paulsen, J., Quijano, K., Reyes, R., Sims, E., Spraggon, G., Stevens, R.C., van den Bedem, H., Velasquez, J., Vincent, J., von Delft, F., Wang, X., West, B., White, A., Wolf, G., Xu, Q., Zagnitko, O., Hodgson, K.O., Wooley, J., Wilson, I.A.(2005) Proteins 58: 971-975
- PubMed: 15657930
- DOI: https://doi.org/10.1002/prot.20360
- Primary Citation of Related Structures:
1VJO
Organizational Affiliation:
The Joint Center for Structural Genomics, The Scripps Research Institute, La Jolla, CA 92037, USA.