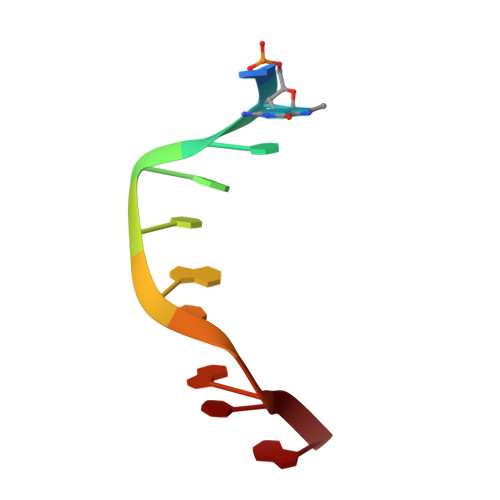
L-nucleotides and 8-methylguanine of d(C1m8G2C3G4C5LG6LC7G8C9G10)2 act cooperatively to promote a left-handed helix under physiological salt conditions.
Cherrak, I., Mauffret, O., Santamaria, F., Hocquet, A., Ghomi, M., Rayner, B., Fermandjian, S.(2003) Nucleic Acids Res 31: 6986-6995
- PubMed: 14627831
- DOI: https://doi.org/10.1093/nar/gkg893
- Primary Citation of Related Structures:
1R4D - PubMed Abstract:
The structure and thermal stability of a hetero chiral decaoligodeoxyribonucleotide duplex d(C1m8 G2C3G4C5LG6LC7G8C9G10)d(C11m8G12C13G14C15LG16LC17G18C19G20) (O1) with two contiguous pairs of enantiomeric 2'-deoxy-L-ribonucleotides (C5LG6L/C15LG16L) at its centre and an 8-methylguanine at position 2/12 was analysed by circular dichroism, NMR and molecular modelling. O1 resolves in a left-handed helical structure already at low salt concentration (0.1 M NaCl). The central L2-sugar portion assumes a B* left-handed conformation (mirror-image of right-handed B-DNA) while its flanking D4-sugar portions adopt the known Z left-handed conformation. The resulting Z4-B2*-Z4 structure (left-handed helix) is the reverse of that of B4-Z2*-B4 (right-handed helix) displayed by the nearly related decaoligodeoxyribonucleotide d(mC1G2mC3G4C5L G6LmC7G8mC9G10)2, at the same low salt concentration (0.1 M NaCl). In the same experimental conditions, d(C1m8G2C3G4C5G6C7G8C9G10)2 (O2), the stereoregular version of O1, resolves into a right-handed B-DNA helix. Thus, both the 8-methylguanine and the enantiomeric step CLpGL at the centre of the molecule are needed to induce left-handed helicity. Remarkably, in the various heterochiral decaoligodeoxyribonucleotides so far analysed by us, when the central CLpGL adopts the B* (respectively Z*) conformation, then the adjacent steps automatically resolves in the Z (respectively B) conformation. This allows a good optimisation of the base-base stackings and base-sugar van der Waals interactions at the ZB*/B*Z (respectively BZ*/Z*B) junctions so that the Z4-B2*-Z4 (respectively B4-Z2*-B4) helix displays a Tm (approximately 65 degrees C) that is only 5 degrees C lower than the one of its homochiral counterpart. Here we anticipate that a large variety of DNA helices can be generated at low salt concentration by manipulating internal factors such as sugar configuration, duplex length, nucleotide composition and base methylation. These helices can constitute powerful tools for structural and biological investigations, especially as they can be used in physiological conditions.
Organizational Affiliation:
Département de Biologie et Pharmacologie Structurales, UMR 8113 CNRS, IGR, 39 rue Camille-Desmoulins, 94805 Villejuif Cedex, France.