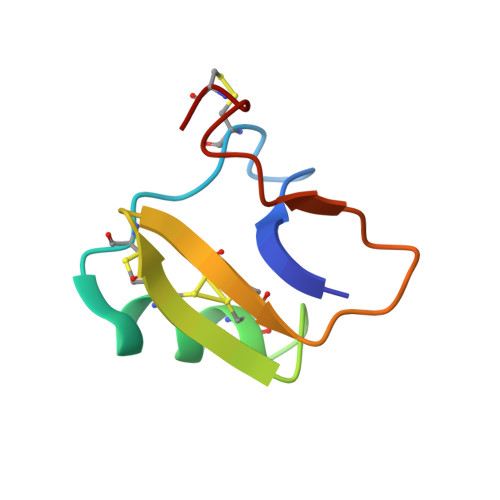
Automatic assignment of NOESY Cross peaks and determination of the protein structure of a new world scorpion neurotoxin Using NOAH/DIAMOD
Xu, Y., Jablonsky, M.J., Jackson, P.L., Braun, W., Krishna, N.R.(2001) J Magn Reson (1969 148: 35-46
- PubMed: 11133274
- DOI: https://doi.org/10.1006/jmre.2000.2220
- Primary Citation of Related Structures:
1NH5 - PubMed Abstract:
The 3D NMR structures of the scorpion neurotoxin, CsE-v5, were determined from the same NOESY spectra with NOAH/DIAMOD, an automated assignment and 3D structure calculation software package, and with a conventional manual assignment combined with a distance geometry/simulated annealing (X-PLOR) refinement method. The NOESY assignments and the 3D structures obtained from the two independent methods were compared in detail. The NOAH/DIAMOD program suite uses feedback filtering and self-correcting distance geometry methods to automatically assign NOESY spectra and to calculate the 3D structure of a protein. NOESY cross peaks were automatically picked using a standard software package and combined with 74 manually assigned NOESY peaks to start the NOAH/DIAMOD calculations. After 63 NOAH/DIAMOD cycles, using REDAC procedures in the last 8 cycles, and final FANTOM constrained energy minimization, a bundle of 20 structures with the smallest target functions has a RMSD of 0.81 A for backbone atoms and 1.11 A for all heavy atoms to the mean structure. Despite some missing chemical shifts of side chain protons, 776 (including 74 manually assigned) of 1130 NOE peaks were unambiguously assigned, 150 peaks have more than one possible assignment compatible with the bundle structures, and only 30 peaks could not be assigned within the given chemical shift tolerance ranges in either the D1 or the D2 dimension. The remaining 174, mainly weak NOE peaks were not compatible with the final 20 best bundle structures at the last NOAH/DIAMOD cycle. The automatically determined structures agree well with the structures determined independently using the conventional method and the same NMR spectra, with the mean RMSD in well-defined regions of 0.84 A for bb and 1.48 A for all heavy atoms from residues 2-5, 18-26, 32-36, and 39-45. This study demonstrates the potential of the NOAH/DIAMOD program suite to automatically assign NMR data for proteins and determine their structure.
Organizational Affiliation:
Department of Human Biological Chemistry and Genetics, Sealy Center for Structural Biology, Galveston, Texas, 77555-1157, USA.