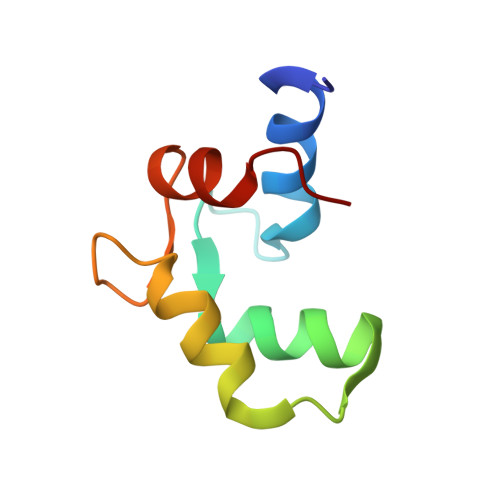
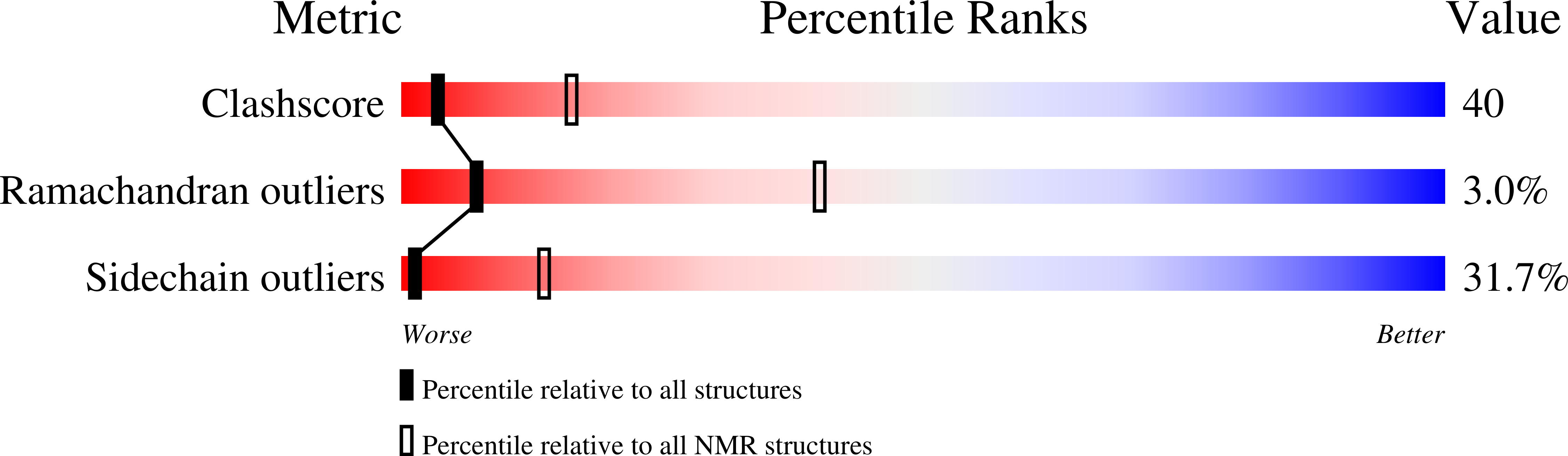Structure of the C-domain of human cardiac troponin C in complex with the Ca2+ sensitizing drug EMD 57033.
Wang, X., Li, M.X., Spyracopoulos, L., Beier, N., Chandra, M., Solaro, R.J., Sykes, B.D.(2001) J Biol Chem 276: 25456-25466
- PubMed: 11320096
- DOI: https://doi.org/10.1074/jbc.M102418200
- Primary Citation of Related Structures:
1IH0 - PubMed Abstract:
Ca(2+) binding to cardiac troponin C (cTnC) triggers contraction in heart muscle. In heart failure, myofilaments response to Ca(2+) are often altered and compounds that sensitize the myofilaments to Ca(2+) possess therapeutic value in this syndrome. One of the most potent and selective Ca(2+) sensitizers is the thiadiazinone derivative EMD 57033, which increases myocardial contractile function both in vivo and in vitro and interacts with cTnC in vitro. We have determined the NMR structure of the 1:1 complex between Ca(2+)-saturated C-domain of human cTnC (cCTnC) and EMD 57033. Favorable hydrophobic interactions between the drug and the protein position EMD 57033 in the hydrophobic cleft of the protein. The drug molecule is orientated such that the chiral group of EMD 57033 fits deep in the hydrophobic pocket and makes several key contacts with the protein. This stereospecific interaction explains why the (-)-enantiomer of EMD 57033 is inactive. Titrations of the cCTnC.EMD 57033 complex with two regions of cardiac troponin I (cTnI(34-71) and cTnI(128-147)) reveal that the drug does not share a common binding epitope with cTnI(128-147) but is completely displaced by cTnI(34-71). These results have important implications for elucidating the mechanism of the Ca(2+) sensitizing effect of EMD 57033 in cardiac muscle contraction.
Organizational Affiliation:
CIHR Group in Protein Structure and Function, Department of Biochemistry, University of Alberta, Edmonton, Alberta T6G 2H7, Canada.