X-Ray Structures of Fv Fragment and its (4-Hydroxy-3-Nitrophenyl)Acetate Complex of Murine B1-8 Antibody
Simon, T., Henrick, K., Hirshberg, M., Winter, G.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| B1-8 FV (LIGHT CHAIN) | A [auth L] | 108 | Mus musculus | Mutation(s): 1 | 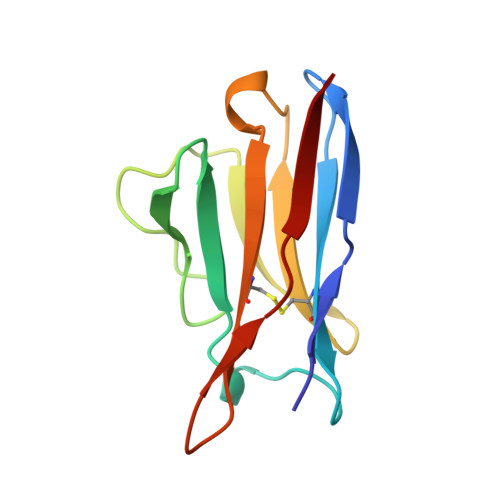 |
UniProt | |||||
Find proteins for P01724 (Mus musculus) Explore P01724 Go to UniProtKB: P01724 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P01724 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| B1-8 FV (HEAVY CHAIN) | B [auth H] | 120 | Mus musculus | Mutation(s): 1 | 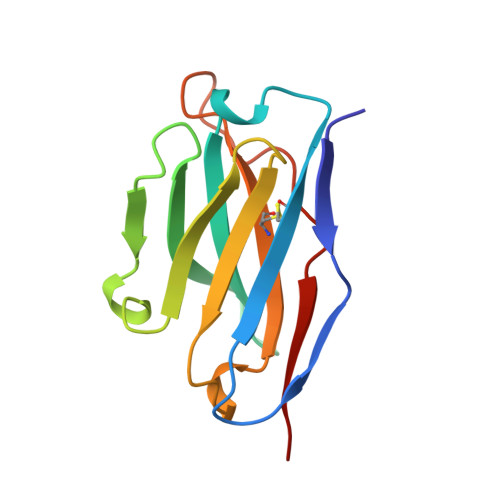 |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 72.03 | α = 90 |
| b = 85.78 | β = 90 |
| c = 37.42 | γ = 90 |
| Software Name | Purpose |
|---|---|
| MOSFLM | data reduction |
| AGROVATA/ROTAVATA | data reduction |
| ALMN/CCP4 | model building |
| PROLSQ | refinement |
| Agrovata | data scaling |
| ROTAVATA | data scaling |
| CCP4 | phasing |