Structural basis of DNA binding by YdaT, a functional equivalent of the CII repressor in the cryptic prophage CP-933P from Escherichia coli O157:H7.
Prolic-Kalinsek, M., Volkov, A.N., Hadzi, S., Van Dyck, J., Bervoets, I., Charlier, D., Loris, R.(2023) Acta Crystallogr D Struct Biol 79: 245-258
- PubMed: 36876434
- DOI: https://doi.org/10.1107/S2059798323001249
- Primary Citation of Related Structures:
8BT1, 8C7K - PubMed Abstract:
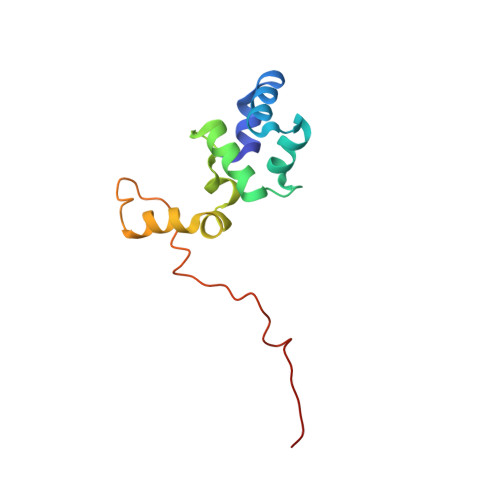
YdaT is a functional equivalent of the CII repressor in certain lambdoid phages and prophages. YdaT from the cryptic prophage CP-933P in the genome of Escherichia coli O157:H7 is functional as a DNA-binding protein and recognizes a 5'-TTGATTN 6 AATCAA-3' inverted repeat. The DNA-binding domain is a helix-turn-helix (HTH)-containing POU domain and is followed by a long α-helix (α6) that forms an antiparallel four-helix bundle, creating a tetramer. The loop between helix α2 and the recognition helix α3 in the HTH motif is unusually long compared with typical HTH motifs, and is highly variable in sequence and length within the YdaT family. The POU domains have a large degree of freedom to move relative to the helix bundle in the free structure, but their orientation becomes fixed upon DNA binding.
Organizational Affiliation:
Structural Biology Brussels, Vrije Universiteit Brussel, Pleinlaan 2, 1050 Brussel, Belgium.