1 H, 13 C, 15 N backbone and side-chain NMR assignments for three MAX effectors from Magnaporthe oryzae.
Lahfa, M., Padilla, A., de Guillen, K., Pissarra, J., Raji, M., Cesari, S., Kroj, T., Gladieux, P., Roumestand, C., Barthe, P.(2022) Biomol NMR Assign 16: 305-309
- PubMed: 35657473
- DOI: https://doi.org/10.1007/s12104-022-10095-2
- Primary Citation of Related Structures:
7ZJY, 7ZK0, 7ZKD - PubMed Abstract:
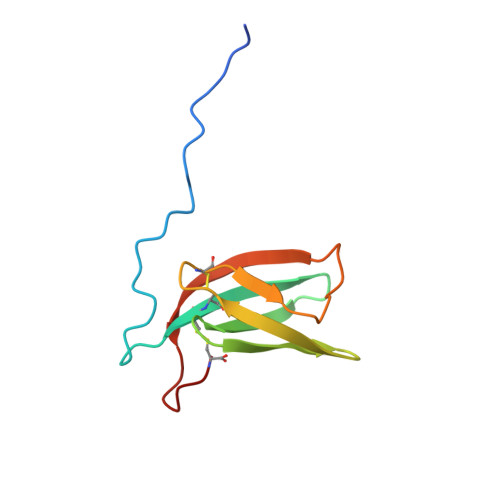
Effectors are small and very diverse proteins secreted by fungi and translocated in plant cells during infection. Among them, MAX effectors (for Magnaporthe Avrs and ToxB) were identified as a family of effectors that share an identical fold topology despite having highly divergent sequences. They are mostly secreted by ascomycetes from the Magnaporthe genus, a fungus that causes the rice blast, a plant disease leading to huge crop losses. As rice is the first source of calories in many countries, especially in Asia and Africa, this constitutes a threat for world food security. Hence, a better understanding of these effectors, including structural and functional characterization, constitutes a strategic milestone in the fight against phytopathogen fungi and may give clues for the development of resistant varieties of rice. We report here the near complete 1 H, 15 N and 13 C NMR resonance assignment of three new putative MAX effectors (MAX47, MAX60 and MAX67). Secondary structure determination using TALOS-N and CSI.3 demonstrates a high content of β-strands in all the three proteins, in agreement with the canonic ß-sandwich structure of MAX effectors. This preliminary study provides foundations for further structural characterization, that will help in turn to improve sequence predictions of other MAX effectors through data mining.
Organizational Affiliation:
Centre de Biologie Structurale, Univ Montpellier, INSERM U1054, CNRS UMR 5048, Montpellier, France.