Structural insight and engineering of a plastic degrading hydrolase Ple629.
Li, Z., Zhao, Y., Wu, P., Wang, H., Li, Q., Gao, J., Qin, H.M., Wei, H., Bornscheuer, U.T., Han, X., Wei, R., Liu, W.(2022) Biochem Biophys Res Commun 626: 100-106
- PubMed: 35981419
- DOI: https://doi.org/10.1016/j.bbrc.2022.07.103
- Primary Citation of Related Structures:
7VPB - PubMed Abstract:
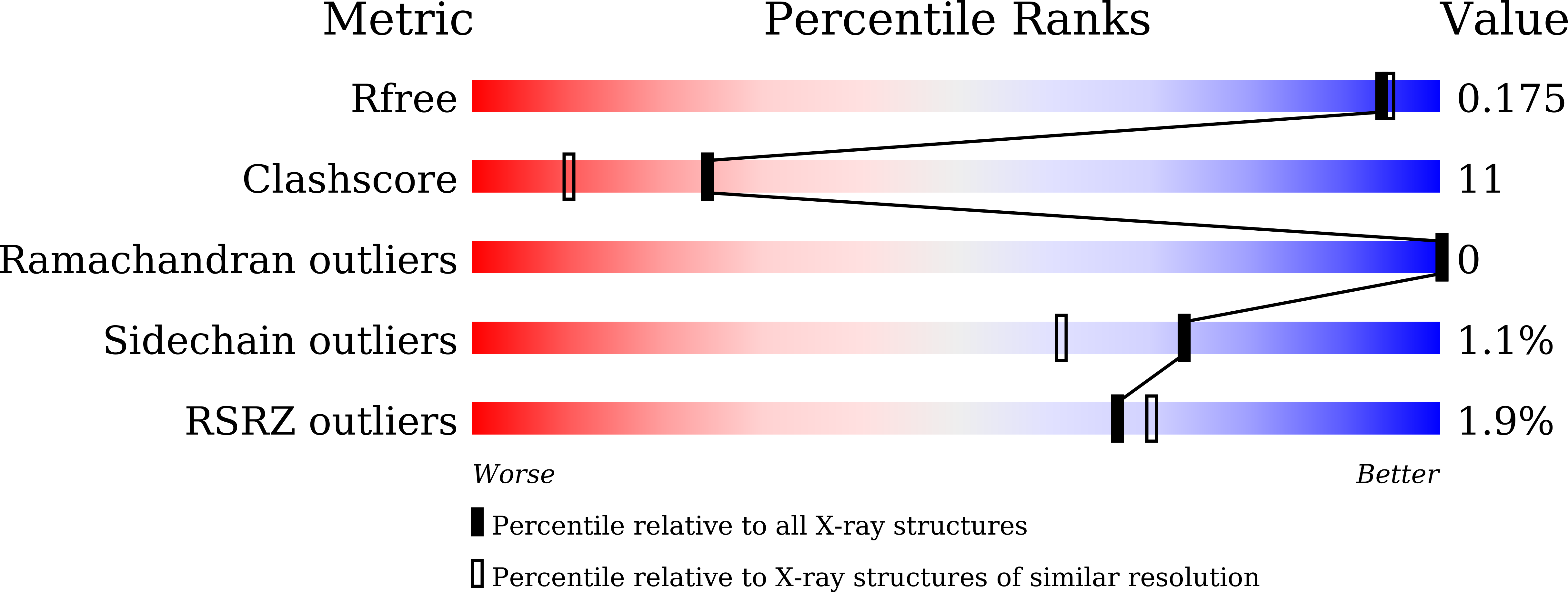
Polyethylene terephthalate (PET) is one of the most abundantly produced synthetic polyesters. The vast number of waste plastics including PET has challenged the waste management sector while also posing a serious threat to the environment due to improper littering. Recently, enzymatic PET degradation has been shown to be a viable option for a circular plastic economy, which can mitigate the plastic pollution. While protein engineering studies on specific PET degradation enzymes such as leaf-branch compost cutinase (LCC), Thermobifida sp. cutinases and Ideonella sakaiensis PETase (IsPETase) have been extensively published, other homologous PET degrading enzymes have received less attention. Ple629 is a polyester hydrolase identified from marine microbial consortium having activity on PET and the bioplastic polybutylene adipate terephthalate (PBAT). In order to explore its catalytic mechanism and improve its potential for PET hydrolysis, we solved its crystal structure in complex with a PET monomer analogue, and validated its structural and mechanistic similarity to known PET hydrolases. By structural comparisons, we identified some hot spot positions described in previous research on protein engineering of PET hydrolases. We substitute these amino acid residues in Ple629, and obtained variants with improved activity and thermo-stability. The most promising variant D226A/S279A exhibited a more than 5.5-fold improved activity on PET nanoparticles than the wild-type enzyme, suggesting its potential applicability in the biotechnological plastic recycling.
Organizational Affiliation:
University of Chinese Academy of Sciences, 19A Yuquan Road, Beijing, 100049, China; Tianjin Institute of Industrial Biotechnology, Chinese Academy of Sciences, Tianjin, 300308, China; National Technology Innovation Center of Synthetic Biology, 32 West 7th Avenue, Tianjin Airport Economic Area, Tianjin, 300308, China.