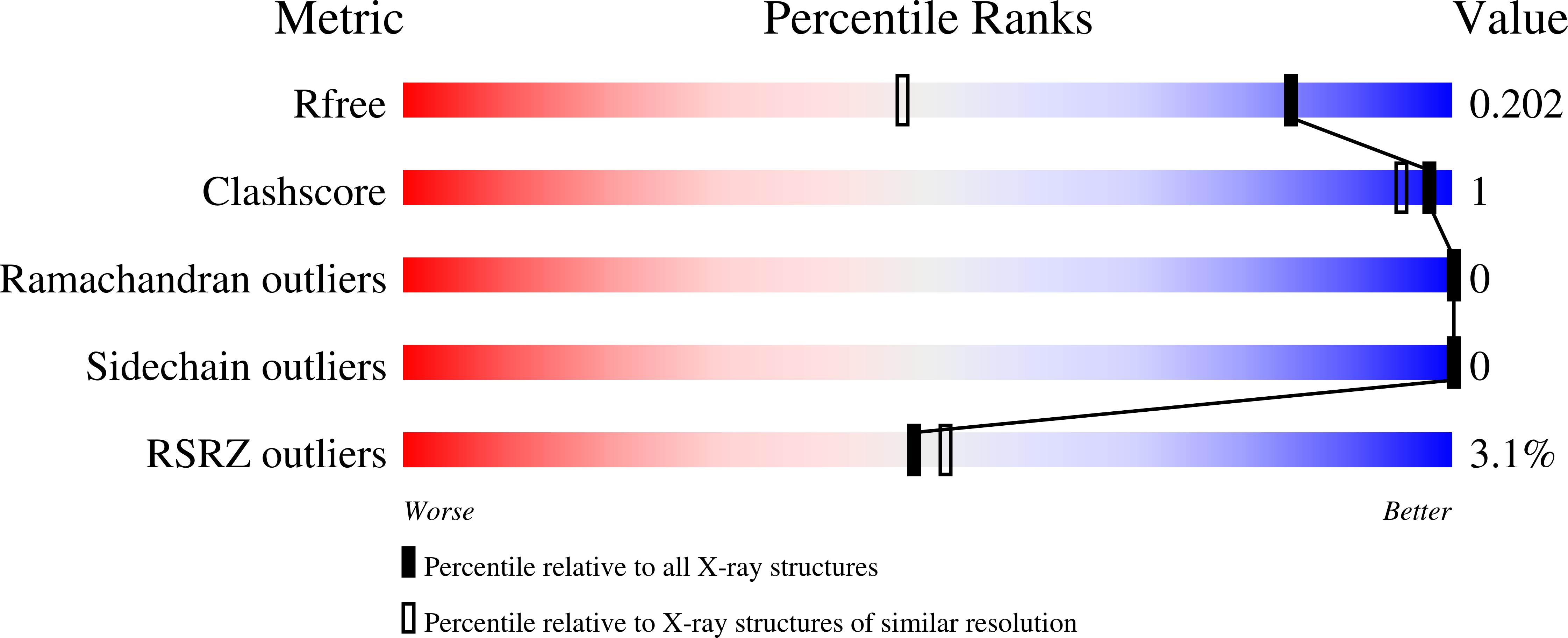
Comparative Performance of PETase as a Function of Reaction Conditions, Substrate Properties, and Product Accumulation.
Erickson, E., Shakespeare, T.J., Bratti, F., Buss, B.L., Graham, R., Hawkins, M.A., Konig, G., Michener, W.E., Miscall, J., Ramirez, K.J., Rorrer, N.A., Zahn, M., Pickford, A.R., McGeehan, J.E., Beckham, G.T.(2022) ChemSusChem 15: e202101932-e202101932
- PubMed: 34587366
- DOI: https://doi.org/10.1002/cssc.202101932
- Primary Citation of Related Structures:
7OSB - PubMed Abstract:
There is keen interest to develop new technologies to recycle the plastic poly(ethylene terephthalate) (PET). To this end, the use of PET-hydrolyzing enzymes has shown promise for PET deconstruction to its monomers, terephthalate (TPA) and ethylene glycol (EG). Here, the Ideonella sakaiensis PETase wild-type enzyme was compared to a previously reported improved variant (W159H/S238F). The thermostability of each enzyme was compared and a 1.45 Å resolution structure of the mutant was described, highlighting changes in the substrate binding cleft compared to the wild-type enzyme. Subsequently, the performance of the wild-type and variant enzyme was compared as a function of temperature, substrate morphology, and reaction mixture composition. These studies showed that reaction temperature had the strongest influence on performance between the two enzymes. It was also shown that both enzymes achieved higher levels of PET conversion for substrates with moderate crystallinity relative to amorphous substrates. Finally, the impact of product accumulation on reaction progress was assessed for the hydrolysis of both PET and bis(2-hydroxyethyl) terephthalate (BHET). Each enzyme displayed different inhibition profiles to mono(2-hydroxyethyl) terephthalate (MHET) and TPA, while both were sensitive to inhibition by EG. Overall, this study highlights the importance of reaction conditions, substrate selection, and product accumulation for catalytic performance of PET-hydrolyzing enzymes, which have implications for enzyme screening in the development of enzyme-based polyester recycling.
Organizational Affiliation:
Renewable Resources and Enabling Sciences Center, National Renewable Energy Laboratory, Golden, CO 80401, United States.