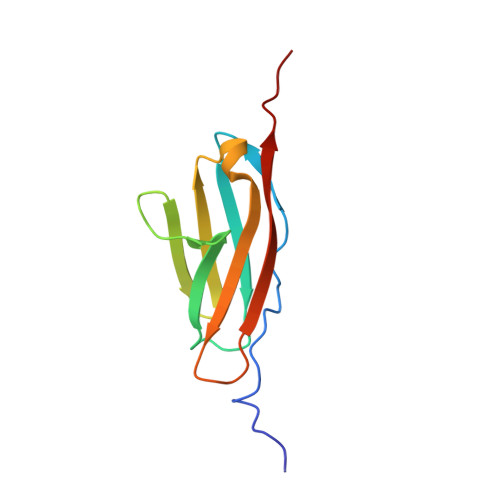
Solution NMR Structure of Titin N2A Region Ig Domain I83 and Its Interaction with Metal Ions.
Kelly, C., Pace, N., Gage, M., Pfuhl, M.(2021) J Mol Biol 433: 166977-166977
- PubMed: 33811919
- DOI: https://doi.org/10.1016/j.jmb.2021.166977
- Primary Citation of Related Structures:
6YJ0 - PubMed Abstract:
Titin, the largest single chain protein known so far, has long been known to play a critical role in passive muscle function but recent studies have highlighted titin's role in active muscle function. One of the key elements in this role is the Ca 2+ -dependent interaction between titin's N2A region and the thin filament. An important element in this interaction is I83, the terminal immunoglobulin domain in the N2A region. There is limited structural information about this domain, but experimental evidence suggests that it plays a critical role in the N2A-actin binding interaction. We now report the solution NMR structure of I83 and characterize its dynamics and metal binding properties in detail. Its structure shows interesting relationships to other I-band Ig domains. Metal binding and dynamics data point towards the way the domain is evolutionarily optimized to interact with neighbouring domains. We also identify a calcium binding site on the N-terminal side of I83, which is expected to impact the interdomain interaction with the I82 domain. Together these results provide a first step towards a better understanding of the physiological effects associated with deletion of most of the I83 domain, as occurs in the mdm mouse model, as well as for future investigations of the N2A region.
Organizational Affiliation:
Department of Chemistry, University of Massachusetts Lowell, Lowell, MA 01854, USA; UMass Movement Center, University of Massachusetts Lowell, Lowell, MA 01854, USA.